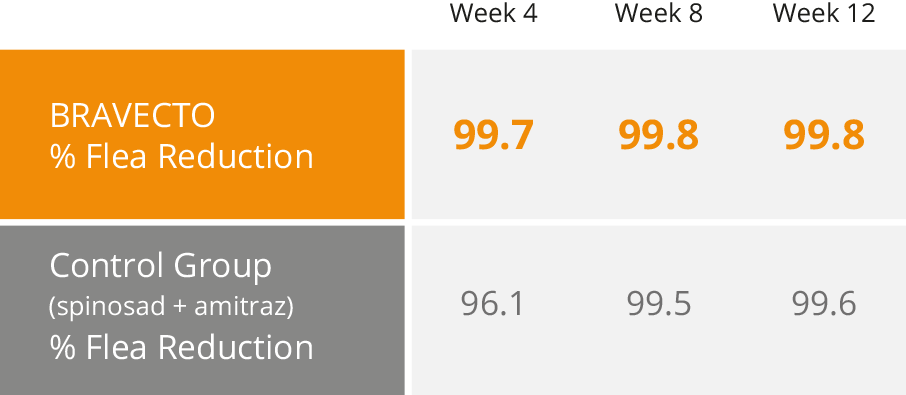
Overall Flea Control with Bravecto vs Spinosad + Amitraz



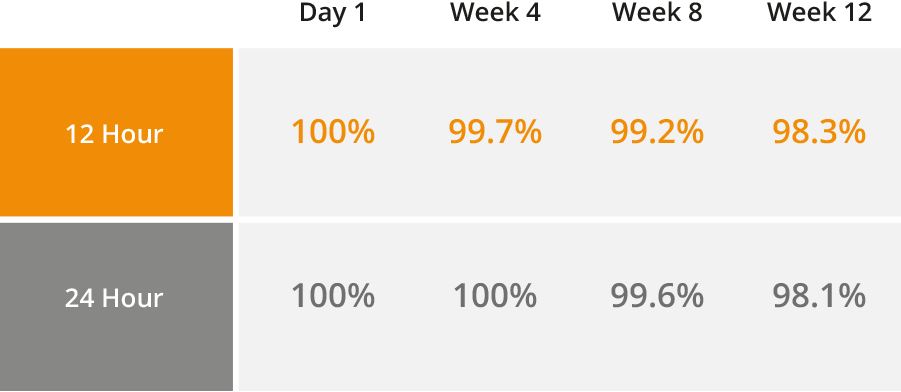
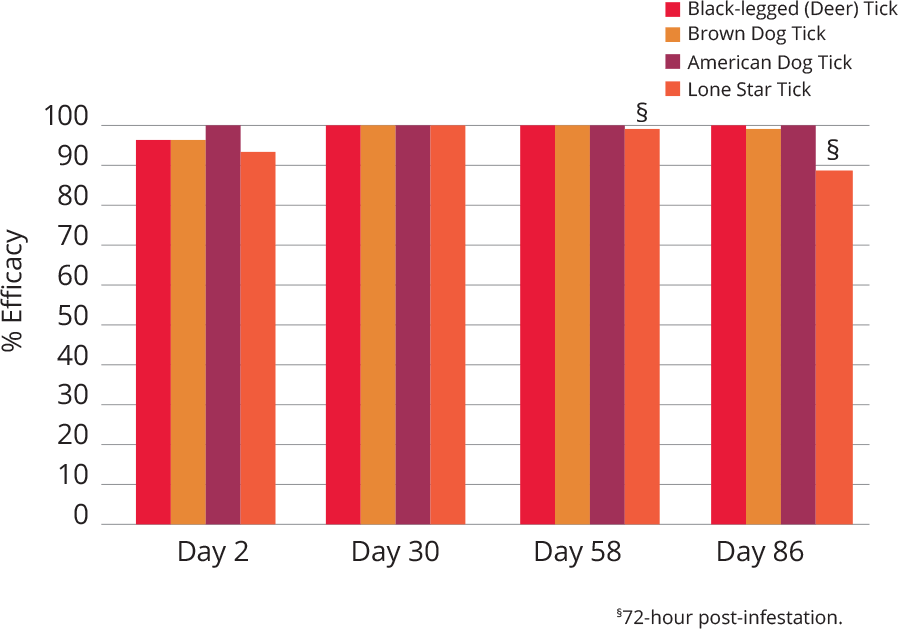
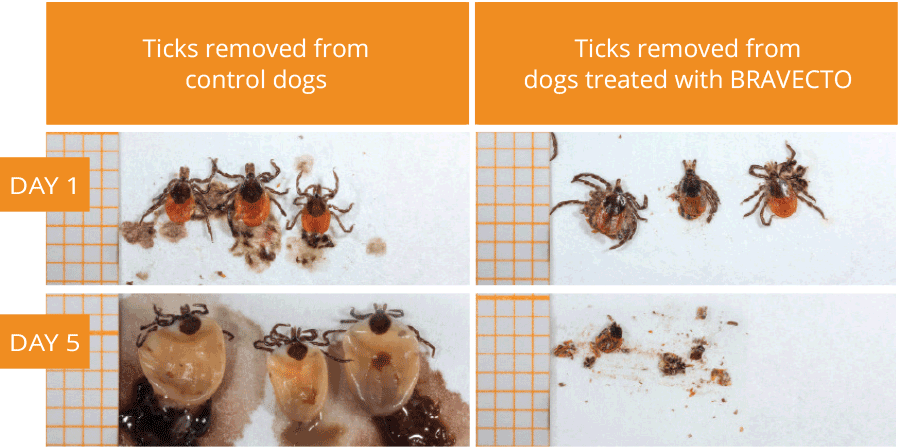
The active ingredient in Bravecto (fluralaner), an ectoparasiticide belonging to the isoxazoline group. The active ingredient is systemically active against fleas and ticks (black-legged tick, American dog tick, Asian longhorned tick, and brown dog tick), delivering protection for 12 weeks*. It also kills lone star ticks for eight weeks. Presented in a highly palatable flavored chew that dogs accept readily.1
INDICATIONS:
Bravecto kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis), and the treatment and control of tick infestations [Ixodes scapularis (black-legged tick), Dermacentor variabilis (American dog tick), Rhipicephalus sanguineus (brown dog tick), and Haemaphysalis longicornis (Asian longhorned tick)] for 12 weeks in dogs and puppies 6 months of age and older, and weighing 4.4 pounds or greater.
Bravecto is also indicated for the treatment and control of Amblyomma americanum (lone star tick) infestations for 8 weeks in dogs and puppies 6 months of age and older, and weighing 4.4 pounds or greater.

Toy dogs
4.4-9.9 LB
112.5 MG

Small dogs
>9.9-22.0 LB
250 MG

Medium dogs
>22.0-44.0 LB
500 MG

Large dogs
>44.0-88.0 LB
1,000 MG

Extra-large dogs
>88.0-123.0† LB
1,400 MG
One chew per weight band1
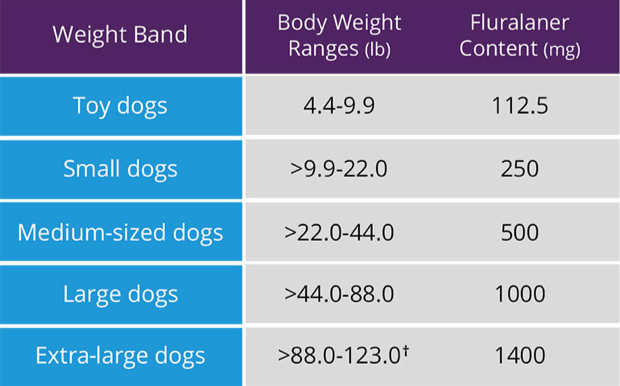
†dogs >123 lbs need appropriate combination of chews


No items to show.


No items to show.

Rhode Island
“The safety profile of Bravecto provides confidence, and the long-lasting efficacy addresses flea infestations in the cat’s home.”

North Carolina
“The benefits of Bravecto’s proven safety and long-lasting efficacy against infestations for dogs and cats.”

New Hampshire
“The efficacious long-lasting protection of Bravecto helps make training and inventory easy.”
DOGS
Bravecto has been evaluated for use in breeding, pregnant, and lactating female dogs.1,2 Bravecto is safe to use and efficacious in adult dogs and puppies over 6 months of age and weighing 4.4 lb. or greater.1,2 It has been tested and found safe in puppies at least 8 to 9 weeks of age at 5x the clinical dose.1,2,17 No adverse effects with Bravecto Chew for Dogs in MDR-1 gene-deficient collies at 3x the recommended dose.18
CATS
Bravecto is safe to use and efficacious in adult cats and kittens over 6 months of age and weighing 2.6. lb. or greater.3 It has been tested and found safe in kittens at least 11 to 13 weeks of age at 5x the clinical dose.3
PROVEN SAFETY
No known contraindications for Bravecto Chew for Dogs and Bravecto Topical Solution for Dogs and Cats.1-3
No problems reported in field trials in dogs and cats that received Bravecto concurrently with other commonly used medication.1-3
In field studies and safety studies, no dogs or cats experienced serious adverse events.1-3,7,8
In margin of safety study, kittens and puppies were dosed at three 8-week intervals.
Contact us for more information about prescribing Bravecto for the dogs and cats in your care.

For technical assistance or to report a suspected adverse drug reaction, contact Merck Animal Health at 1-800-224-5318.
This site is intended for veterinary professionals. Visit our website for pet parents.
*BRAVECTO kills fleas and prevents flea infestations. BRAVECTO (fluralaner) Chews for Dogs kills ticks (black-legged tick, American dog tick, brown dog tick, and Asian longhorned tick) for 12 weeks. BRAVECTO Chews also kills lone star ticks for 8 weeks. BRAVECTO (fluralaner topical solution) for Dogs kills ticks (black-legged tick, American dog tick, and brown dog tick) for 12 weeks. BRAVECTO Topical Solution for Dogs also kills lone star ticks for 8 weeks. BRAVECTO (fluralaner topical solution) for Cats kills ticks (black-legged tick and Asian longhorned tick) for 12 weeks. BRAVECTO Topical Solution for Cats also kills American dog ticks for 8 weeks
BRAVECTO 1-MONTH (fluralaner) Chews: indicated for dogs 8 weeks of age and older. The most commonly reported adverse reactions include itching, diarrhea, vomiting, decreased appetite, elevated ALT, lethargy, and weight loss. BRAVECTO 1-MONTH is not effective against A. americanum in puppies less than 6 months of age. BRAVECTO (fluralaner) Chews for Dogs: The most commonly reported adverse reactions include vomiting, lethargy, diarrhea, anorexia and pruritus. In some cases, adverse events have been reported following use in breeding females. BRAVECTO (fluralaner topical solution) for Dogs: The most commonly reported adverse reactions include vomiting, hair loss, diarrhea, lethargy, decreased appetite, and moist dermatitis/rash. BRAVECTO (fluralaner topical solution) for Cats: The most commonly reported adverse reactions include vomiting, itching, diarrhea, hair loss, decreased appetite, lethargy, and scabs/ulcerated lesions. BRAVECTO Topical Solution for Cats is not effective against American dog ticks beyond 8 weeks of dosing. BRAVECTO PLUS (fluralaner and moxidectin topical solution) for Cats: The most commonly reported adverse reactions include vomiting, hair loss, itching, diarrhea, lethargy, dry skin, elevated ALT, and hypersalivation. BRAVECTO PLUS has not been shown to be effective for 2 months in kittens less than 6 months of age. Use with caution in cats that are heartworm positive. The effectiveness of BRAVECTO PLUS to prevent heartworm disease after bathing or water immersion has not been evaluated.
BRAVECTO Chews and Topical Solution for dogs have not been shown to be effective for 12-weeks’ duration in puppies or kittens less than 6 months of age. BRAVECTO Chews and Topical Solution for Dogs are not effective against the lone star tick beyond 8 weeks of dosing. BRAVECTO Topical Solution for Dogs and Cats and BRAVECTO PLUS for Cats are for topical use only. Avoid oral ingestion. The safety of BRAVECTO Topical Solution for Cats and BRAVECTO PLUS have not been established in breeding, pregnant and lactating cats.
All BRAVECTO products contain fluralaner, which is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders. Neurologic adverse reactions have been reported in cats receiving isoxazoline class drugs, even in cats without a history of neurologic disorders. Use with caution in cats with a history of neurologic disorders.
References:
1. Bravecto Chew for Dogs [prescribing information]. Madison, NJ: Merck Animal Health; 2014.
2. Bravecto Topical Solution for Dogs [prescribing information]. Madison, NJ: Merck Animal Health; 2016.
3. Bravecto Topical Solution for Cats [prescribing information]. Madison, NJ: Merck Animal Health; 2016.
4. Rohdich N, Roepke RKA, Zschiesche E. A randomized, blinded, controlled and multi-centered field study comparing the efficacy and safety of Bravecto™ (fluralaner) against Frontline™ (fipronil) in flea- and tick-infested dogs. Parasites & Vectors. 2014;7:83.
5. Beck S, Schein E, Baldermann C, von Samson-Himmelstjerna G, Kohn B. Tick infestation and tick prophylaxis in dogs in the area of Berlin/Brandenburg – results of a questionnaire study. Berl Munch Tierarztl Wochenschr. 2013;126(1-2):69-76.
6. Kidd L, Breitschwerdt EB. Transmission times and prevention of tick-borne diseases in dogs. Compend Contin Educ Pract Vet. 2003;(25)10:742-751.
7. Freedom of Information Summary, NADA 141-426. Approved May 15, 2014.
8. Freedom of Information Summary, NADA 141-459. Approved 2016.
9. Gassel M, Wolf C, Noack S, Williams H, Ilg T. The novel isoxazoline ectoparasiticide fluralaner: selective inhibition of arthropod γ-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem Mol Biol. 2014;45:111-124.
10. Williams H, Demeler J, Taenzler J, Roepke RK, Zshiesche E, Heckeroth AR. A quantitative evaluation of the extent of fluralaner uptake by ticks (Ixodes ricinus, Ixodes scapularis) in fluralaner (Bravecto™) treated vs. untreated dogs using the parameters tick weight and coxal index. Parasites & Vectors. 2015;8:352.
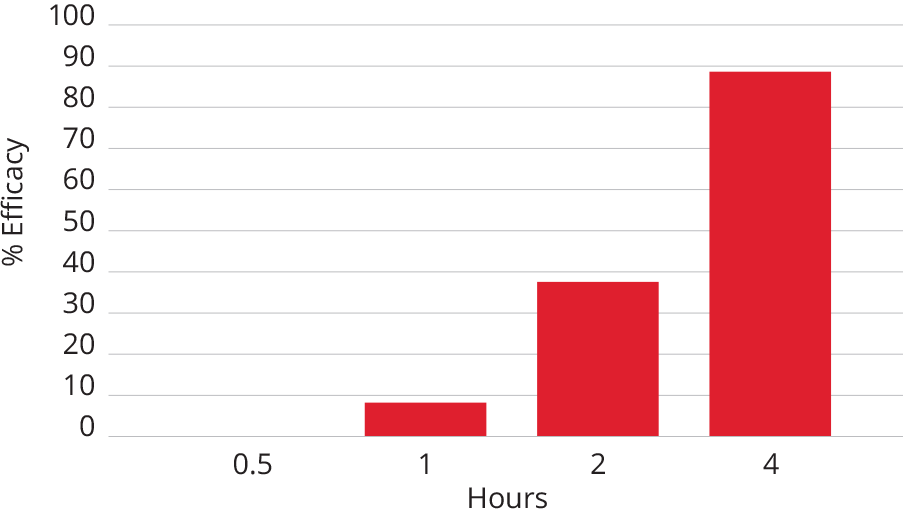
11. Taenzler J, Wengenmayer C, Williams H, et al. Onset of activity of fluralaner (Bravecto™) against Ctenocephalides felis on dogs. Parasites & Vectors. 2014;7:567.
12. Meadows C, Guerino F, Sun F. A randomized, blinded, controlled USA field study to assess the use of fluralaner tablets in controlling flea infestations. Parasites & Vectors. 2014;7:375.
13. CAPCvet.org. Accessed July 5, 2016.
14. Data on File, Merck Animal Health.
15. Taenzler J, Liebenberg J, Roepke RKA, Heckeroth AR. Prevention of transmission of Babesia canis by Dermacentor reticulatus ticks to dogs treated orally with fluralaner chewable tablets (Bravecto™). Parasites & Vectors. 2015;8:305.
16. Wengenmayer C, Williams H, Zschiesche E, et al. The speed of kill of fluralaner (Bravecto™) against lxodes ricinus ticks on dogs. Parasites & Vectors. 2014;7:525.
17. Walther FM, Allan MJ, Roepke RKA, Nuernberger MC. Safety of fluralaner chewable tablets (Bravecto™), a novel systemic antiparasitic drug, in dogs after oral administration. Parasites & Vectors. 2014;7:87.
18. Walther FM, Allan MJ, Roepke RKA, Nuernberger MC. Safety of fluralaner, a novel systemic antiparasitic drug, in MDR1(-/-) collies after oral administration. Parasites & Vectors. 2014;7:86.
19. Burgio et al. Parasites & Vectors (2016) 9:626.
20. Meadows et al, Parasites & Vectors, (2017) 10:36.
21. Meadows et al, Parasites & Vectors, (2017) 10:37.