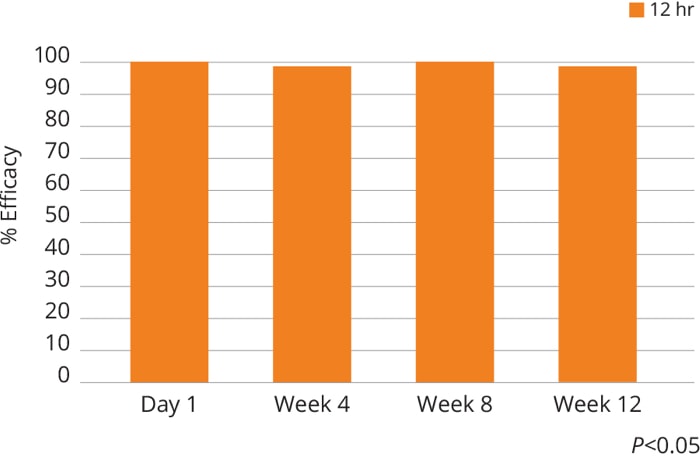
Flea Efficacy for Cats for Up to 12 weeks3,8
- Erythema
- Papules
- Crusts
- Alopecia
- Scales
- Excoriation
Frontline® Plus is a registered trademark of Merial.



A fast-acting flea and tick protection for cats. Bravecto (fluralaner) is the feline flea control medication that is easy for pet owners to administer. Flea and tick prevention lasts up to 12 weeks* with just a single dose.
INDICATIONS:
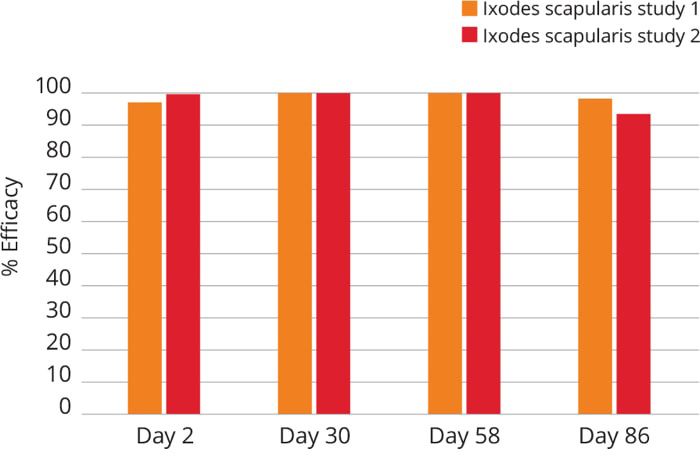
Bravecto kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of Ixodes scapularis (black-legged tick) and Haemaphysalis longicornis (Asian longhorned tick) infestations for 12 weeks in cats and kittens 6 months of age and older, and weighing 2.6 pounds or greater.
Bravecto is also indicated for the treatment and control of Dermacentor variabilis (American dog tick) infestations for 8 weeks in cats and kittens 6 months of age and older, and weighing 2.6 pounds or greater.

Small cats
2.6—6.2 lbs

Medium cats
>6.2—13.8 lbs

Large cats
>13.8—27.5 lbs§
One application per weight
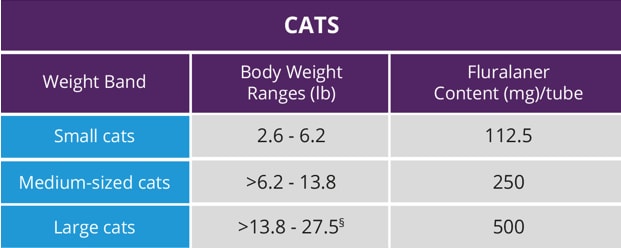
§Cats over 27.0 lb. should be administered the appropriate combination of tubes.


Frontline® Plus is a registered trademark of Merial.
No items to show.
No items to show.

Tennessee
“Bravecto goes a long way in solving clients’ flea and tick problems, so they’ll keep coming back to the clinic.”

Illinois
“Bravecto is a popular choice among pet owners and the compliance numbers back it up.”

Texas
“Bravecto gives pet owners the joy of a flea and tick-free household.”
DOGS
Bravecto has been evaluated for use in breeding, pregnant, and lactating female dogs.1,2 Bravecto is safe to use and efficacious in adult dogs and puppies over 6 months of age and weighing 4.4 lb. or greater.1,2 It has been tested and found safe in puppies at least 8 to 9 weeks of age at 5x the clinical dose.1,2,17 No adverse effects with Bravecto Chew for Dogs in MDR-1 gene-deficient collies at 3x the recommended dose.18
CATS
Bravecto is safe to use and efficacious in adult cats and kittens over 6 months of age and weighing 2.6. lb. or greater.3 It has been tested and found safe in kittens at least 11 to 13 weeks of age at 5x the clinical dose.3
PROVEN SAFETY
No known contraindications for Bravecto Chew for Dogs and Bravecto Topical Solution for Dogs and Cats.1-3
No problems reported in field trials in dogs and cats that received Bravecto concurrently with other commonly used medication.1-3
Bravecto has not been shown to be effective for 12 weeks1 duration in puppies or kittens less than 6 months of age.
In field studies and safety studies, no dogs or cats experienced serious adverse events.1-3,7,8
In margin of safety study, kittens and puppies were dosed at three 8-week intervals.
Contact us for more information about prescribing Bravecto for the dogs and cats in your care.

For technical assistance or to report a suspected adverse drug reaction, contact Merck Animal Health at
1-800-224-5318.
This site is intended for veterinary professionals. Visit our website for pet parents.
*BRAVECTO kills fleas and prevents flea infestations. BRAVECTO (fluralaner) Chews for Dogs kills ticks (black-legged tick, American dog tick, brown dog tick, and Asian longhorned tick) for 12 weeks. BRAVECTO Chews also kills lone star ticks for 8 weeks. BRAVECTO (fluralaner topical solution) for Dogs kills ticks (black-legged tick, American dog tick, and brown dog tick) for 12 weeks. BRAVECTO Topical Solution for Dogs also kills lone star ticks for 8 weeks. BRAVECTO (fluralaner topical solution) for Cats kills ticks (black-legged tick and Asian longhorned tick) for 12 weeks. BRAVECTO Topical Solution for Cats also kills American dog ticks for 8 weeks
BRAVECTO 1-MONTH (fluralaner) Chews: indicated for dogs 8 weeks of age and older. The most commonly reported adverse reactions include itching, diarrhea, vomiting, decreased appetite, elevated ALT, lethargy, and weight loss. BRAVECTO 1-MONTH is not effective against A. americanum in puppies less than 6 months of age. BRAVECTO (fluralaner) Chews for Dogs: The most commonly reported adverse reactions include vomiting, lethargy, diarrhea, anorexia and pruritus. In some cases, adverse events have been reported following use in breeding females. BRAVECTO (fluralaner topical solution) for Dogs: The most commonly reported adverse reactions include vomiting, hair loss, diarrhea, lethargy, decreased appetite, and moist dermatitis/rash. BRAVECTO (fluralaner topical solution) for Cats: The most commonly reported adverse reactions include vomiting, itching, diarrhea, hair loss, decreased appetite, lethargy, and scabs/ulcerated lesions. BRAVECTO Topical Solution for Cats is not effective against American dog ticks beyond 8 weeks of dosing. BRAVECTO PLUS (fluralaner and moxidectin topical solution) for Cats: The most commonly reported adverse reactions include vomiting, hair loss, itching, diarrhea, lethargy, dry skin, elevated ALT, and hypersalivation. BRAVECTO PLUS has not been shown to be effective for 2 months in kittens less than 6 months of age. Use with caution in cats that are heartworm positive. The effectiveness of BRAVECTO PLUS to prevent heartworm disease after bathing or water immersion has not been evaluated.
BRAVECTO Chews and Topical Solution for dogs have not been shown to be effective for 12-weeks’ duration in puppies or kittens less than 6 months of age. BRAVECTO Chews and Topical Solution for Dogs are not effective against the lone star tick beyond 8 weeks of dosing. BRAVECTO Topical Solution for Dogs and Cats and BRAVECTO PLUS for Cats are for topical use only. Avoid oral ingestion. The safety of BRAVECTO Topical Solution for Cats and BRAVECTO PLUS have not been established in breeding, pregnant and lactating cats.
All BRAVECTO products contain fluralaner, which is a member of the isoxazoline class. This class has been associated with neurologic adverse reactions including tremors, ataxia, and seizures. Seizures have been reported in dogs receiving isoxazoline class drugs, even in dogs without a history of seizures. Use with caution in dogs with a history of seizures or neurologic disorders. Neurologic adverse reactions have been reported in cats receiving isoxazoline class drugs, even in cats without a history of neurologic disorders. Use with caution in cats with a history of neurologic disorders.
All trademarks are the property of their respective owners.