Nobivac® NXT Canine Flu H3N2
Discover the first and only canine influenza vaccine to use RNA particle technology to generate an efficient, comprehensive immune response.

Discover the next (NXT) generation of protection. Nobivac® NXT Canine Flu H3N2 is a groundbreaking new way to safeguard dogs against the ongoing threat of canine flu with revolutionary RNA particle technology.1
Features & Benefits
RNA particle technology
- RNA particles stimulate the immune system to attack the H3N2 virus without the use of adjuvants or live organisms
Multi-layered Immunity
- Enhances the body’s natural processes to induce innate, humoral, and cellular immunity
Smaller dose
- A low-volume 0.5 mL dose provides a more gentle vaccine experience.
No extraneous material
- Nobivac® NXT-level protection in a preservative-free, adjuvant-free, thimerosal-free formulation.
How It Works
Discover the science behind Nobivac® NXT-level RNA particle technology. See how this revolutionary new way of delivering protection provides an exciting new option for you and your patients.
Downloads

NOBIVAC® NXT Canine Flu H3N2
DELIVER NXT-LEVEL IMMUNITY
Fight canine influenza virus (CIV) with revolutionary RNA particle technology in a non-adjuvanted, low-volume vaccine.

Addressing Canine Influenza
Your guide to the prevention and management of canine influenza virus
Use this brochure to help guide your practice in the identification, containment, and prevention of CIV outbreaks.
No items to show.
Product Specifications
Administration
- For primary immunization, administer 1 dose (0.5 mL) subcutaneously as early as 8 weeks of age followed by a second dose 3 to 4 weeks later
Indication
- Effective for the vaccination of healthy dogs 8 weeks of age or older against canine influenza virus H3N2
Supplied
- 25 doses per tray
Safety
- Safety has been demonstrated in dogs 8 weeks of age and older
Uncompromising Safety and Efficacy
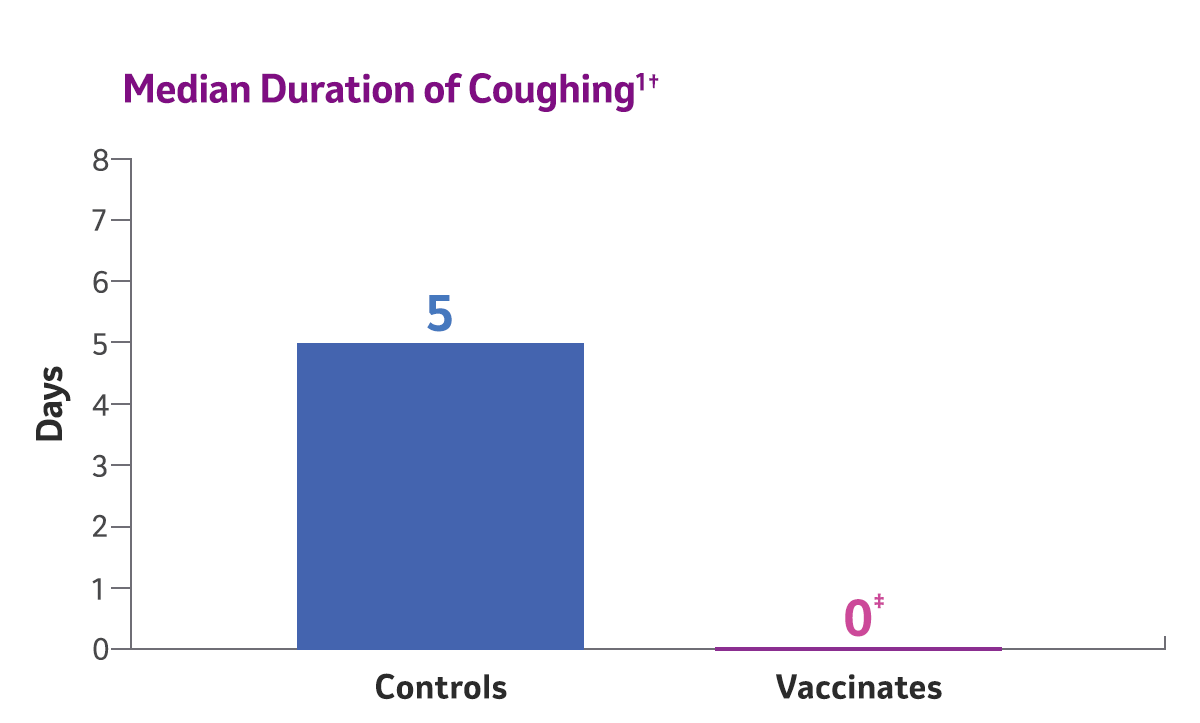
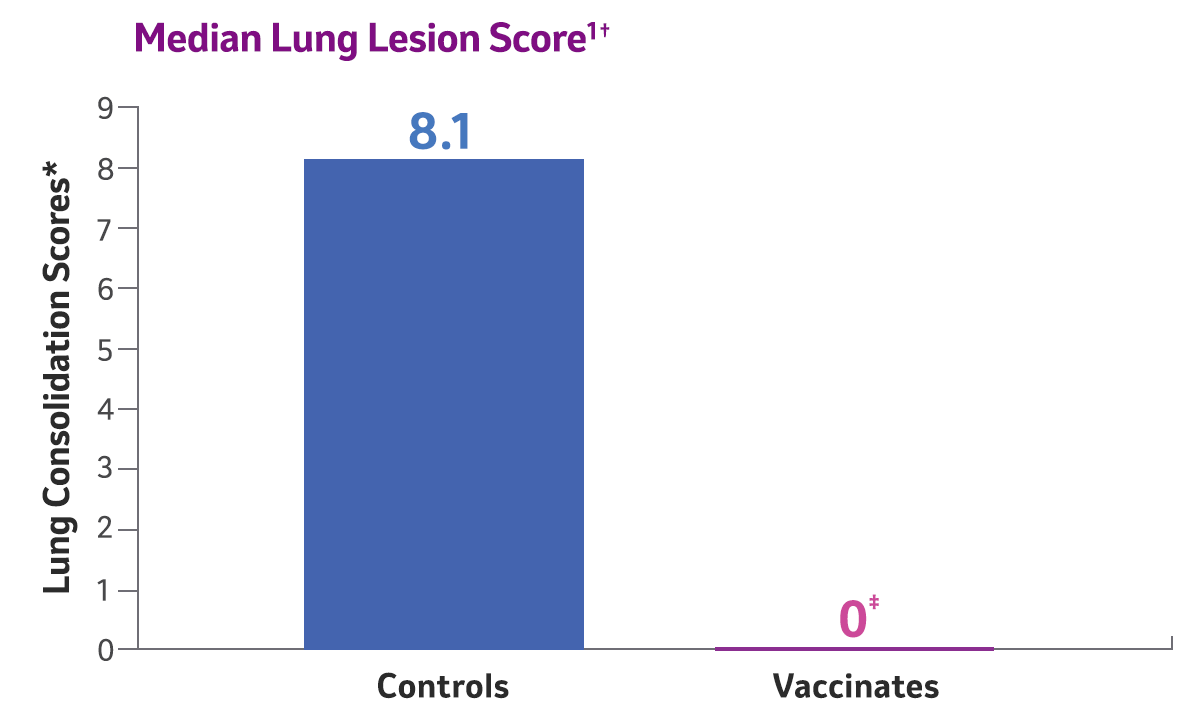
*The standardized method of evaluating efficacy of canine influenza vaccines is based on lung lobe consolidation.2
† Two doses of Nobivac® NXT Canine Flu H3N2 were administered to 8-week-old puppies, subcutaneously, 3 weeks apart. Challenge was conducted 2 weeks post 2nd vaccination. Clinical observations were recorded for 10 days post-challenge, and nasal swabs were collected for 10 consecutive days to determine viral shedding. Lungs were evaluated 10 days following challenge.
‡ P<0.0001
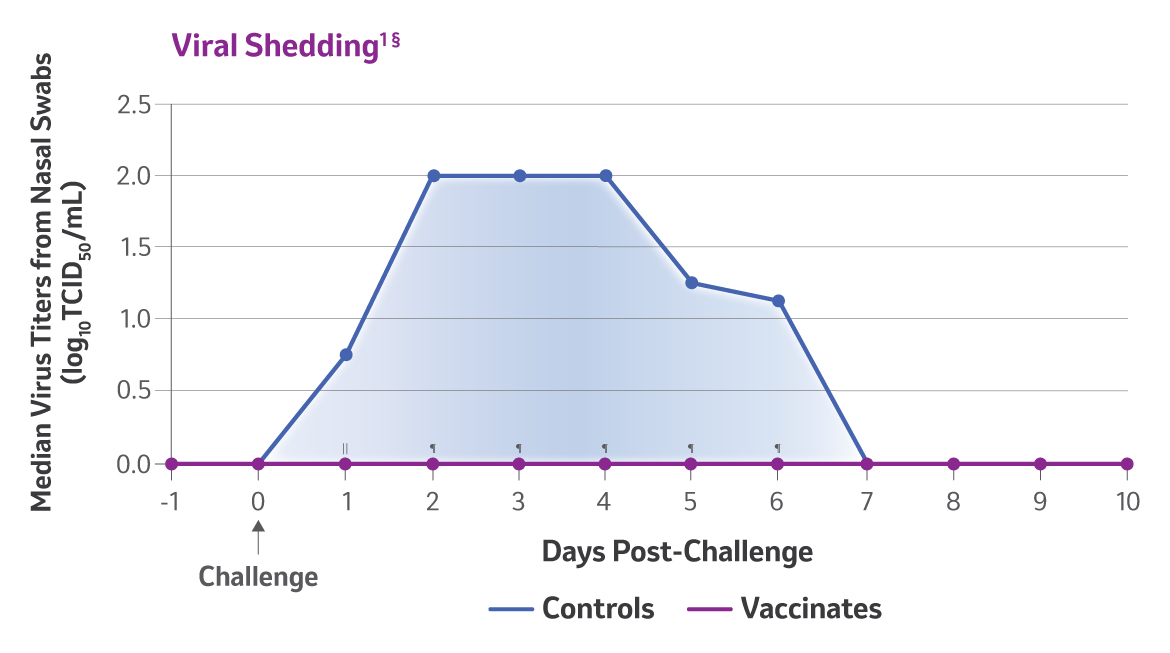
§Two doses of Nobivac® NXT Canine Flu H3N2 were administered to 8-week-old puppies, subcutaneously, 3 weeks apart. Challenge was conducted 2 weeks post 2nd vaccination. Clinical observations were recorded for 10 days post-challenge, and nasal swabs were collected for 10 consecutive days to determine viral shedding. Lungs were evaluated 10 days following challenge.
|| Magnitude of viral shedding on Day 1 – P=0.0001
¶ Overall duration of viral shedding and magnitude of viral shedding – P<0.0001
A field safety study proved Nobivac® NXT Canine Flu H3N2 to be well tolerated in a wide range of canine patients1
- 1,301 doses administered to 654 dogs of various breeds
- Dogs ranged in age from 8 weeks to 15 years
- Lethargy was the most common adverse event (1.6%)
Disease Information
Canine influenza virus (CIV) causes a respiratory infection in dogs that is also known as dog flu. The infection is very contagious to other dogs. Laboratory surveillance indicates the H3N2 strain of CIV as the critical strain of concern.1
Transmission
- Direct contact (licking, nuzzling)
- Indirect (coughing or sneezing)
- Contaminated surfaces such as food and water bowls, cages
- Human contact
Clinical Signs
- Coughing (dry or moist)
- Sneezing
- Fever
- Nasal discharge
- Lack of energy
- Loss of appetite
Risk Factors
- Dogs that come from shelters, rescue centers, breeding kennels, or pet stores
- Boarding at a kennel or doggie daycare
- Visiting groomers, dog parks, or engaging with other dogs
- Dogs that participate in events/competitions
- Dogs that travel
- Dogs of veterinary staff that may be exposed through contaminated fomites
- Sharing an elevator or other confined space with other dogs

NOBIVAC® NXT-GENERATION PROTECTION
Learn more about Nobivac® NXT technology, the latest vaccine platform from Nobivac®.
References
- Data on file. Merck Animal Health.
- Deshpande MS, Jirjis FF, Tubbs AL, et al. Evaluation of the efficacy of a canine influenza virus H3N8 vaccine in dogs following experimental challenge. Vet Therapeut. 2009;10(3): 103-112.



