

The FIRST veterinary insulin
FDA-approved for both dogs
and cats2,3

Vetsulin® For Life –
Diabetes Vet Nurse Program
Sign up today for this eBook series that helps veterinary nurses
and technicians work with pet parents to manage pet diabetes.
PROMOTING HEALTHY PETS WITH VETSULIN®
Vetsulin® (porcine insulin zinc suspension) is the first FDA-approved veterinary product for the management of canine and feline diabetes mellitus.
Features
- Intermediate-acting insulin
- Controls blood glucose levels
- Lessens clinical signs of diabetes
- Two delivery options
Indications:
Vetsulin is an insulin indicated for the reduction of hyperglycemia and hyperglycemia-associated clinical signs in dogs and cats with diabetes mellitus.
Two Options
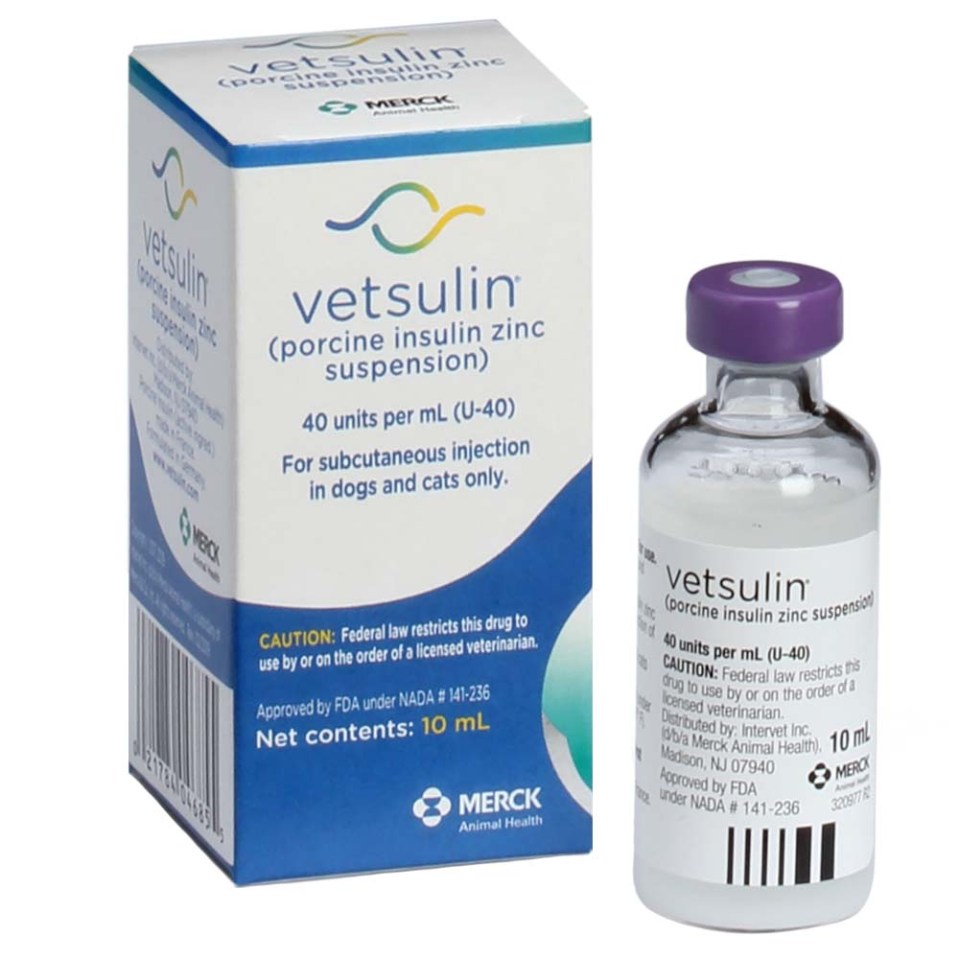
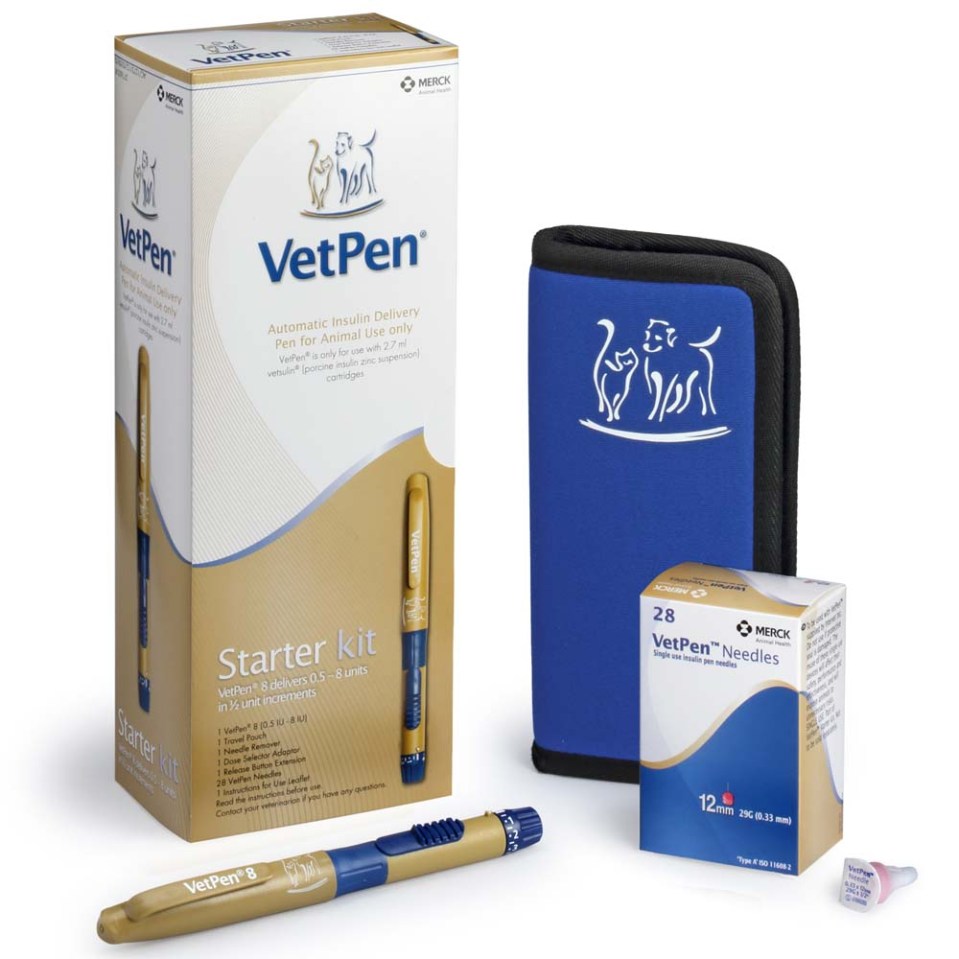
Pharmacokinetics
As a lente insulin, Vetsulin is classified as an intermediate-acting insulin. It is an aqueous suspension of 40 IU/mL of highly purified porcine insulin, consisting of 35% amorphous and 65% crystalline zinc insulin.
Unlike human insulin, porcine insulin has the same amino acid sequence as canine insulin, making it less likely for dogs to develop anti-insulin antibodies.
In Dogs4
The amorphous fraction will reach its maximum effect approximately 4 hours following subcutaneous administration, and its effects last for about 8 hours thereafter. The effect is maintained by the crystalline fraction, which has a slower onset of action and peak effects around 11 hours following injection. The duration of activity of Vetsulin generally ranges from 14–24 hours in dogs.
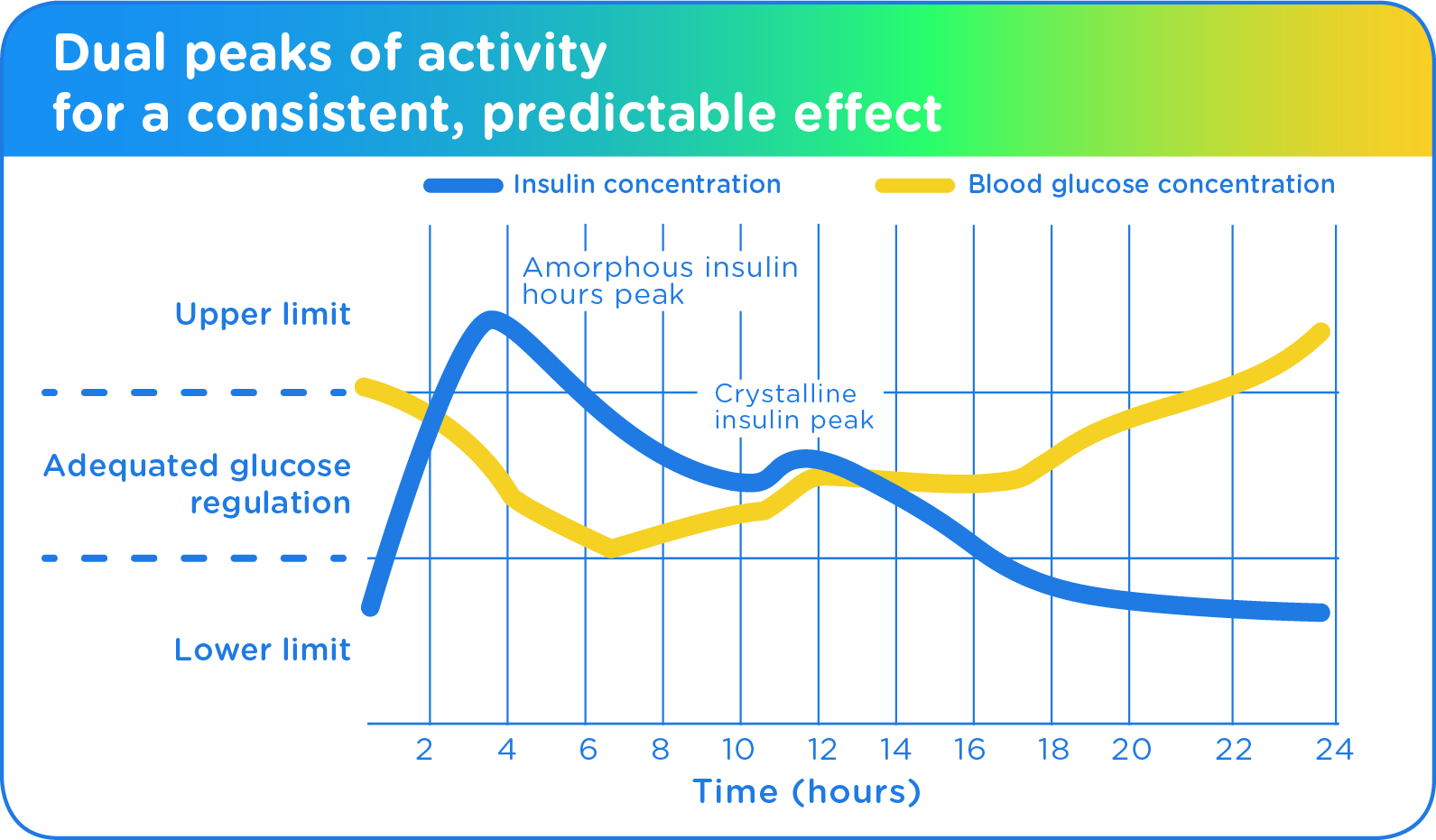
In Cats5
The peak activity following subcutaneous administration of Vetsulin in cats occurs between 1.5 and 8 hours (with an average of about 4 hours), and the duration of activity varies between 8 and 12 hours.1
Efficacy & Safety
As the first FDA-approved insulin for pets, Vetsulin® is proven safe and effective for both cats and dogs with diabetes.
In Dogs
Vetsulin achieved glycemic control and reduced clinical signs in dogs with diabetes in FDA-licensing study.3
- Treated dogs demonstrated significantly lower blood glucose levels following treatment with Vetsulin. Investigators reported adequate glycemic control an average of 81% of time during the study period.
- Treated dogs demonstrated reduced hyperglycemic clinical signs following treatment with Vetsulin.
- 94%, 96%, and 83% of test dogs experienced a reduction in polyuria, polydipsia, and ketonuria, respectively.
In Cats
Vetsulin reduced all major diabetes indicators and hyperglycemic signs to within normal range, and was well tolerated in a US pivotal study of 78 cats.2,3
- FDA-licensing study demonstrated that Vetsulin was well tolerated.2,3
- Clinical signs of hypoglycemia, which were generally mild, occurred in only 16.7% of test cats.
- All cases resolved with symptomatic treatment or dose adjustment, with 5% of the cats in remission.
- Cats were initially started on 1 to 2 IU of Vetsulin per injection twice daily, evaluated with blood glucose curves and clinical signs, and necessary adjustments made.
- At the end of the study, the mean Vetsulin dose was 3.3 IU per injection BID with a maximum dose of 8 IU per injection twice daily.
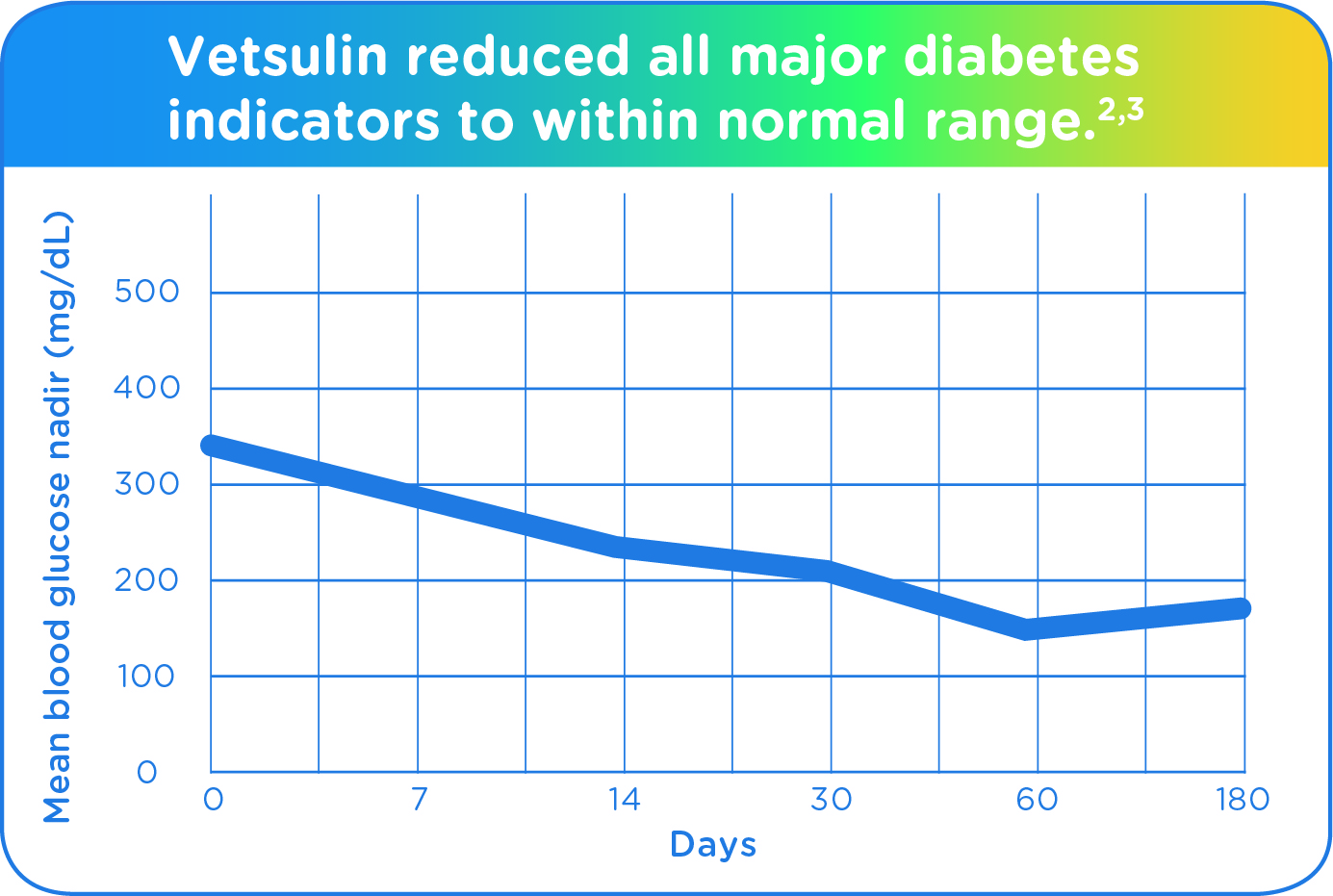
Average blood glucose nadir on Days 7, 14, 30, 60, and 180 was reduced compared to Day 0 (pretreatment initiation).
- 75% of cats had a mean blood glucose level <300 mg/dL by Day 60 following Vetsulin treatment.
- 72% of cats had a mean glucose nadir <200 mg/dL by Day 60 after Vetsulin treatment.
- The mean blood glucose nadir was 145.7 mg/dL by Day 60.
- Fructosamine was significantly reduced on Days 30, 60, and 180 compared to Day 0.
Storage & Handling
Ensure proper usage and storage for all Vetsulin® (porcine insulin zinc suspension) products.
Insulin Storage
Protect from light and avoid high temperatures, keep refrigerated at 36°F to 46°F (2°C to 8°C). Do not freeze insulin because this will denature the insulin. Store insulin vials upright to avoid crystallization around the stopper, but when VetPen® is loaded it can be stored on its side.
Use insulin contents within 42 days of first puncture.
Before Delivery
Vetsulin is a suspension, with active ingredients present in the precipitate and in the clear supernatant.
Before administering, all insulin should be shaken thoroughly until a homogeneous, uniformly milky suspension is obtained. Any foam formed during shaking should be allowed to disperse before the product is used.
Product should be shaken thoroughly if clumps or white particles are seen. Do not use if visible clumps or white particles persist after shaking.
Syringe & Needle Handling & Disposal
Clients should be advised not to reuse insulin syringes or VetPen® needles. Reuse of any syringe can cause bacterial infection; as well as silicone contamination from the syringe causing a white participate that can interfere with the biological activity of the insulin.
The used syringes and VetPen needles should be disposed of appropriately in a sharps/biohazard receptacle.
Delivery Information
View complete instructions for preparation, delivery and disposal to ensure proper amount of insulin is given.
Vetsulin® U-40 Insulin Syringe Administration
VetPen®
Administration
VetPen®
Administration Videos
The Diabetes Resource for Pet Parents
Vetsulin.com is a great source of information for pet parents to learn about diabetes, including proper treatment and monitoring to help their pets live normal, healthy lives.
Help Your Practice Manage Diabetes Mellitus
View and download resources and tools that will assist your hospital, inform your team, and help with clients.

Blood Glucose Curve Generator
Create a blood glucose curve to monitor and evaluate diabetes treatments.

Client Discharge Form
Create a customized, printable form for clients about their new diagnosis.

Diabetes Resources
Access online tools and more to support staff and pet parents.
No items to show.
*Vetsulin® is sold as Caninsulin® outside of the United States.
Important Safety Information:
Vetsulin® should not be used in dogs known to have a systemic allergy to pork or pork products. Vetsulin is contraindicated during periods of hypoglycemia. Keep out of reach of children. As with all insulin products, careful patient monitoring for hypoglycemia and hyperglycemia is essential to attain and maintain adequate glycemic control and prevent associated complications. Overdosage can result in profound hypoglycemia and death. The safety and effectiveness of Vetsulin in puppies, breeding, pregnant, and lactating dogs has not been evaluated. See package insert for full information regarding contraindications, warnings, and precautions.
VetPen® User Safety Warning: For use in animals only. Keep out of the reach of children. Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for at least 15 minutes. Accidental injection may cause clinical hypoglycemia. In case of accidental injection, seek medical attention immediately. Exposure to the product may induce a local or systemic allergic reaction in sensitized individuals.
References:
1. Martin GJ, Rand JS. Pharmacology of a 40 IU/ml porcine lente insulin preparation in diabetic cats: findings during the first week and after 5 or 9 weeks of therapy. J Feline Med Surg. 2001;3(1):23–30. 2. Vetsulin® (porcine insulin zinc suspension) [Freedom of Information Summary]. Millsboro, DE: Intervet Inc.; 2008. 3. Data on file, Merck Animal Health. 4. Graham PA, Nash AS, McKellar QA. Pharmacokinetics of porcine insulin zinc suspension in diabetic dogs. J Small Anim Pract. 1997;38(10):434–438. 5. Martin GJ, Rand JS. Pharmacokinetic and Pharmacodynamic Study of Caninsulin in Cats with Diabetes Mellitus. 2000: Internal Study Report. 6. Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. 3rd ed. St. Louis, MO: Saunders; 2004:539–579. 7. Tennant B, ed. BSAVA Small Animal Formulary. 4th ed. Gloucestershire, UK: British Small Animal Veterinary Association; 2002. 8. Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. 3rd ed. St. Louis, MO: Saunders; 2004:486–538. 9. Reusch C. Feline diabetes mellitus. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, MO: Saunders; 2010:1796–1816. 10. Nelson RW. Canine diabetes mellitus. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, MO: Saunders; 2010:1782–1796. 11. Burgaud S, Riant S, Piau N. Comparative laboratory evaluation of dose delivery using a veterinary insulin pen. In: Proceedings of the WSAVA/FECAVA/BSAVA congress; 12–15 April 2012; Birmingham, UK. Abstract 121. 12. Burgaud S, Guillot R, Harnois-Milon G. Clinical evaluation of a veterinary insulin pen in diabetic dogs. In: Proceedings of the WSAVA/ FECAVA/BSAVA congress; 12–15 April 2012; Birmingham, UK. Abstract 122. 13. Burgaud S, Guillot R, Harnois-Milon G. Clinical evaluation of a veterinary insulin pen in diabetic cats. In: Proceedings of the WSAVA/FECAVA/BSAVA congress; 12–15 April 2012; Birmingham, UK. Abstract 45. 14. Davison LJ, Walding B, Herrtage ME, Catchpole B. Anti-insulin antibodies in diabetic dogs before and after treatment with different insulin preparations. J Vet Intern Med. 2008;22:1317-1325. 15. Banfield State of Pet Health 2016 Report. p 12-13.
