
NOBIVAC EDGE® DAPPv
NOBIVAC EDGE® DAPPv is a 0.5mL, combined vaccination approved for protection against canine distemper virus, adenovirus type 1 (hepatitis), canine parainfluenza virus, and canine parvovirus.
NOBIVAC EDGE® DAPPv provides protection against four known viruses in a 0.5ML vaccine
Canine Distemper Virus, Canine Adenovirus 1 (hepatitis), Canine Parainfluenza Virus, Canine Parvovirus (modified live viruses).
- Protects against all known strains of CPV, including CPV-2c2,3
- High antigenic mass (titer), low passage parvovirus vaccine
- Canine vaccine containing CPV-2b, one of the most prevalent field strains of parvovirus
- Protects against adenovirus type 1 that causes hepatitis in dogs
- Provides disease coverage for commonly spread canine viruses, distemper, adenovirus types 1, parainfluenza and parvovirus in 1 formula
Indications:
Shown to be effective for the vaccination against distemper, adenovirus type 1 (hepatitis), parainfluenza, and parvovirus in one formula. Recommended for use in healthy dogs 6 weeks of age or older.
Efficacy & Comparisons
NOBIVAC EDGE® DAPPv IS A SAFE CHOICE
- Safety confirmed in 346-dog field study1
- Proven safe across a variety of dog breeds and ages
ADMINISTRATION AND DOSAGE
- Subcutaneous injection
- Primary vaccination may begin as early as 6 weeks
- Repeat dose 2 to 4 weeks later. Two doses are required for primary immunization.
- Historically, annual revaccination has been recommended for this product. The need for this booster has not been established.
- Available in a 25 x 0.5 mL dose presentation
ALSO AVAILABLE IN OTHER FORMULATIONS
DISEASE INFORMATION
MORBIDITY THREATS
- Signs vary from a slight fever to death.
- The mortality rate ranges from 10%–30%.
- Severely infected dogs may develop convulsions from forebrain damage.
- Paresis, ataxia and central blindness have also been described.
- In ~25% of recovered dogs, bilateral corneal opacity develops.
SPREADING DISEASE
- The virus is spread in the feces, urine, blood, saliva, and nasal discharge of infected dogs.
- Contracted through the mouth or nose, where it replicates in the tonsils.
- The incubation period is 4 to 7 days.
DIAGNOSIS
Abrupt onset of illness and bleeding suggest ICH, although clinical evidence is not always sufficient to differentiate ICH from distemper (see Canine Distemper).
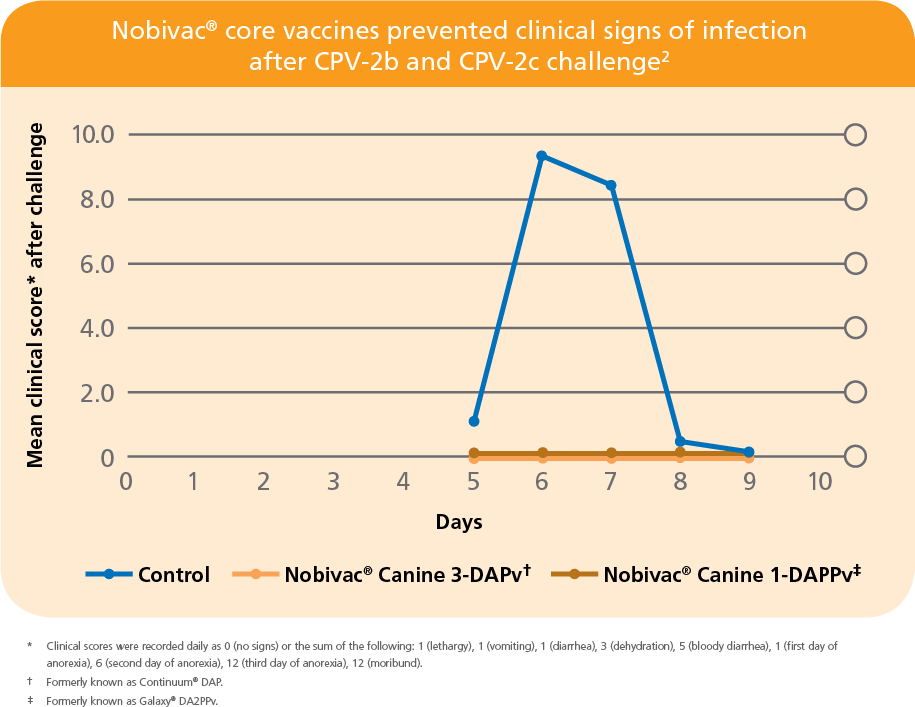
PROVEN IN AN INDEPENDENT STUDY BY LARSON AND SCHULTZ2
- No clinical signs of disease were seen in Nobivac-vaccinated puppies after challenge with mixed CPV-2b and CPV-2c strains2
- First published data showing protection against CPV-2c challenge2
- All vaccinated puppies were protected from disease
- All control puppies developed disease and 50% died or were euthanized
D–CANINE DISTEMPER VIRUS (CDV)
- Onderstepoort-type strain in Nobivac® vaccines provides a high level of safety1
A–CANINE ADENOVIRUS TYPE 2 (CAV-2)
- Protection against CAV-1 (hepatitis) without the side effects associated with modified live CAV-1 vaccines1
P–CANINE PARAINFLUENZA VIRUS (CPIV)
- Systemic administration stimulates a strong IgG response1
Professional Resources and Educational Materials
Keep your clinic and staff informed and aware of diseases and outbreaks.

Quick Guide to Lepto
eBook
A handy guide to protect dogs and prevent the spread of leptospirosis.

Nobivac® Social Media Kits
Digital Assets
Use these ready-to-share posts to educate pet parents and drive business to your clinic for preventative care.

AAHA Canine Vaccination Guidelines
SOP
In-depth information about canine vaccinations and veterinary best practices.
No items to show.



