

Feline 1-HCP+FeLV
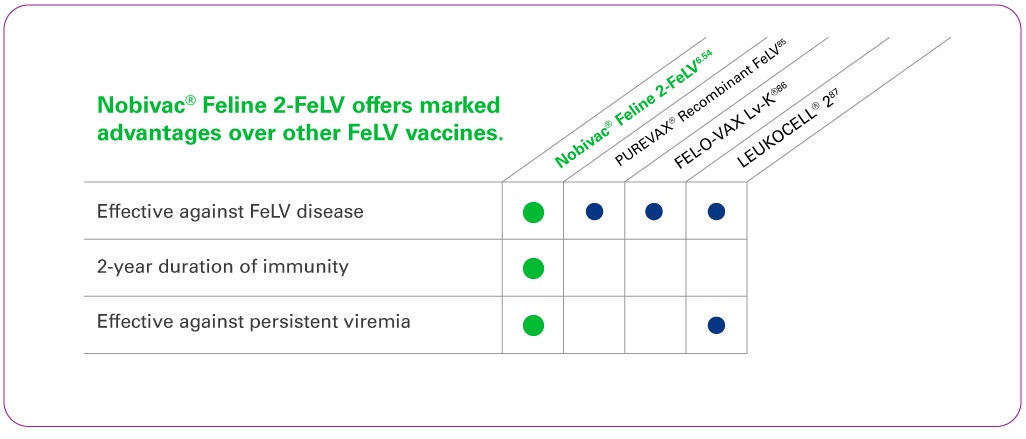
Only Nobivac® FeLV combination vaccines provide two-year duration of immunity for feline leukemia virus in addition to being effective against feline rhinotracheitis, calicivirus, and panleukopenia.
FELINE 1-HCP+FeLV provides combination virus protection for cats
Nobivac® Feline 1-HCP+FeLV is a combination vaccine uniting the benefits of a modified live core vaccine with a killed FeLV vaccine in one vaccination.
- A quality core vaccine shown to be effective for vaccination of healthy cats 9 weeks of age or older against feline rhinotracheitis, calici, panleukopenia, and feline leukemia viruses, which provides a 2-year duration of immunity against feline leukemia virus.
- Nobivac® Feline 1-HCP has been shown to block the replication of canine parvovirus (CPV) in cats1
- Evidence shows that CPV-2a, CPV-2b, and CPV-2c isolates can replicate in cats, producing clinical signs of feline panleukopenia61-63
- Only Nobivac® FeLV vaccines are shown to be effective against persistent viremia for 2 years after vaccination.64
- Superior Efficacy demonstrated vs. multiple FeLV vaccines65, 66
- Nobivac® FeLV vaccines are the ONLY USDA-licensed vaccines that offer 2-Year Duration of Immunity (DOI) (Ideal for adherence to AAFP guidelines).1, 64, 67
- The ONLY vaccine to demonstrate efficacy over the long-term in a co-mingling study mimicking real life conditions1
- The optimal choice for both indoor and outdoor cats of all ages and for all cats under 1 year of age
Indications:
Shown to be effective for vaccination of healthy cats 9 weeks of age or older against feline rhinotracheitis, calici, panleukopenia, and feline leukemia viruses. Duration of Immunity against feline leukemia virus is at least 2 years. Also shown to be effective against persistent viremia in cats exposed to virulent leukemia virus.
EFFICACY & COMPARISONS
ONLY Nobivac® FeLV vaccines offer a 2-year DOI.64
FeLV vaccine was shown to be effective against persistent viremia.1, 69
FELINE 1-HCP+FELV IS A SAFE CHOICE
- 98.8% reaction-free in field safety studies
- 99.99% reaction-free in ongoing surveillance involving millions of doses1
- Study showed no signs of inflammation or adjuvant at Day 21 after subcutaneous vaccination1
- Two-year FeLV DOI means fewer vaccinations
ADMINISTRATION AND DOSAGE
- Subcutaneous or intramuscular injection
- Initial 1 mL dose administered at 9 weeks of age or older
- Second 1 mL dose administered 3 to 4 weeks later
- Historically, annual revaccination has been recommended for this product. The need for this booster has not been established. For more information on revaccination frequency, in general or in the face of maternal antibody, consult your veterinarian or the manufacturer.
- Available in a 25 x 1mL dose presentation
ALSO AVAILABLE IN OTHER FORMULATIONS
DISEASE INFORMATION
Professional Resources and Educational Materials
Keep your clinic and staff informed and aware of diseases and outbreaks.

Feline Vaccination Guide
Brochure
A guide to help your pet parents understand common feline diseases and the importance of customizing vaccination for their pet’s unique lifestyle.

Feline Leukemia Virus
Brochure
Educate your clients about FeLV with this easy to share brochure.
No items to show.



