



Introducing the first oral Bordetella bronchiseptica and canine parainfluenza virus vaccine with proprietary Immuno-Mist-R™ technology

Our proprietary formulation and patented
Immuno-Mist-R™ technology make it possible.
Bordetella bronchiseptica (avirulent live culture), canine parainfluenza virus (modified live virus)

Two of the most common respiratory pathogens vaccinated against are Bordetella bronchiseptica (Bb) and canine parainfluenza virus (Pi). However, an oral combination Bb and Pi vaccine has never been possible—until now.
Vaccinate with a new level of confidence and ease:
- Groundbreaking Immuno-Mist-R™ technology dramatically increases mucosal surface area contact
- Broad mucosal coverage induces an effective immune response
- Oral administration offers comfort for dogs and convenience for staff
Twist, mist, and go!
The first-of-its-kind Immuno-Mist-R™ applicator delivers the vaccine in a fine mist
for greater mucosal coverage than traditional oral vaccines.
Vaccinate against Bb and Pi, together, in 3 easy steps.*

Step 1
Draw vaccine into Luer-lock
syringe and remove the needle.

Step 2
Twist on Immuno-Mist-R™
applicator until tight.

Step 3
Mist toward the back of the mouth
for can’t-miss coverage.
*Refer to product insert for complete instructions.
How to administer

- 1-mL dose reconstituted prior to administration
- To be administered orally
- Luer-lock syringe & patented Immuno-Mist-R™ applicator included
- Approved for dogs 7 weeks of age or older
How it works

- Patented Immuno-Mist-R™ provides broad mucous membrane surface coverage
- Stimulates an effective antibody response
- Mucosal antibodies attack the pathogens before they enter the body
Efficacy
5X SHORTER DURATION OF COUGHING RELATED TO B. BRONCHISEPTICA COMPARED TO CONTROLS1
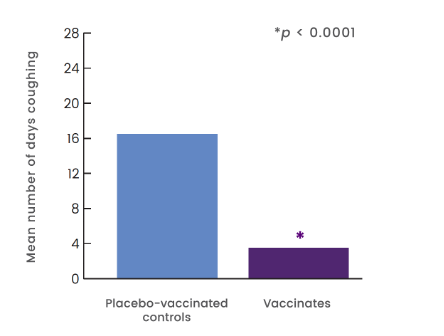
- 21 dogs were given a 1-mL dose of Nobivac® Intra-Trac® Oral BbPi orally at Day 0
- 21 dogs were given placebo orally at Day 0
- Dogs were challenged 5 weeks post vaccination
NOBIVAC® INTRA-TRAC®3 INTRANASAL OFFERS ADVANTAGES OVER ORAL AND INJECTABLE VACCINES
4x shorter duration of shedding related to CPiV compared to controls1
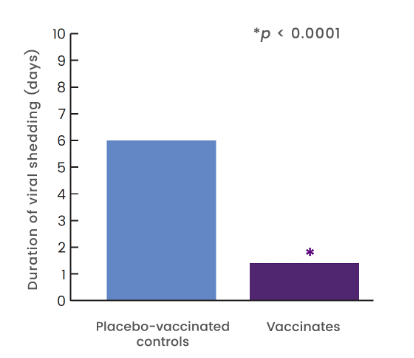
- 20 dogs were given 1-mL dose of Nobivac® Intra-Trac® Oral BbPi
- orally at Day 0
- 19 dogs were given placebo orally at Day 0
- Dogs were challenged 3 weeks post vaccination
Significantly reduced long-term bacterial
shedding compared to controls1
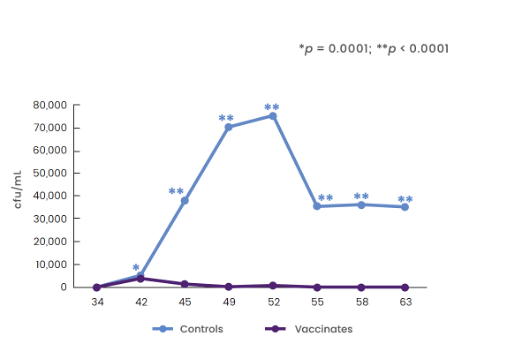
- 21 dogs were given 1-mL dose of Nobivac® Intra-Trac® Oral BbPi
- orally at Day 0
- 21 dogs were given placebo orally at Day 0
- Dogs were challenged 5 weeks post vaccination (Day 36)
Reference:
1. Data on file. Merck Animal Health.
Disease Information
Resources

Nobivac® Intra-Trac® Oral BbPi
DIGITAL DETAILER
Download the full digital detailer for an interactive look at this revolutionary vaccine.


