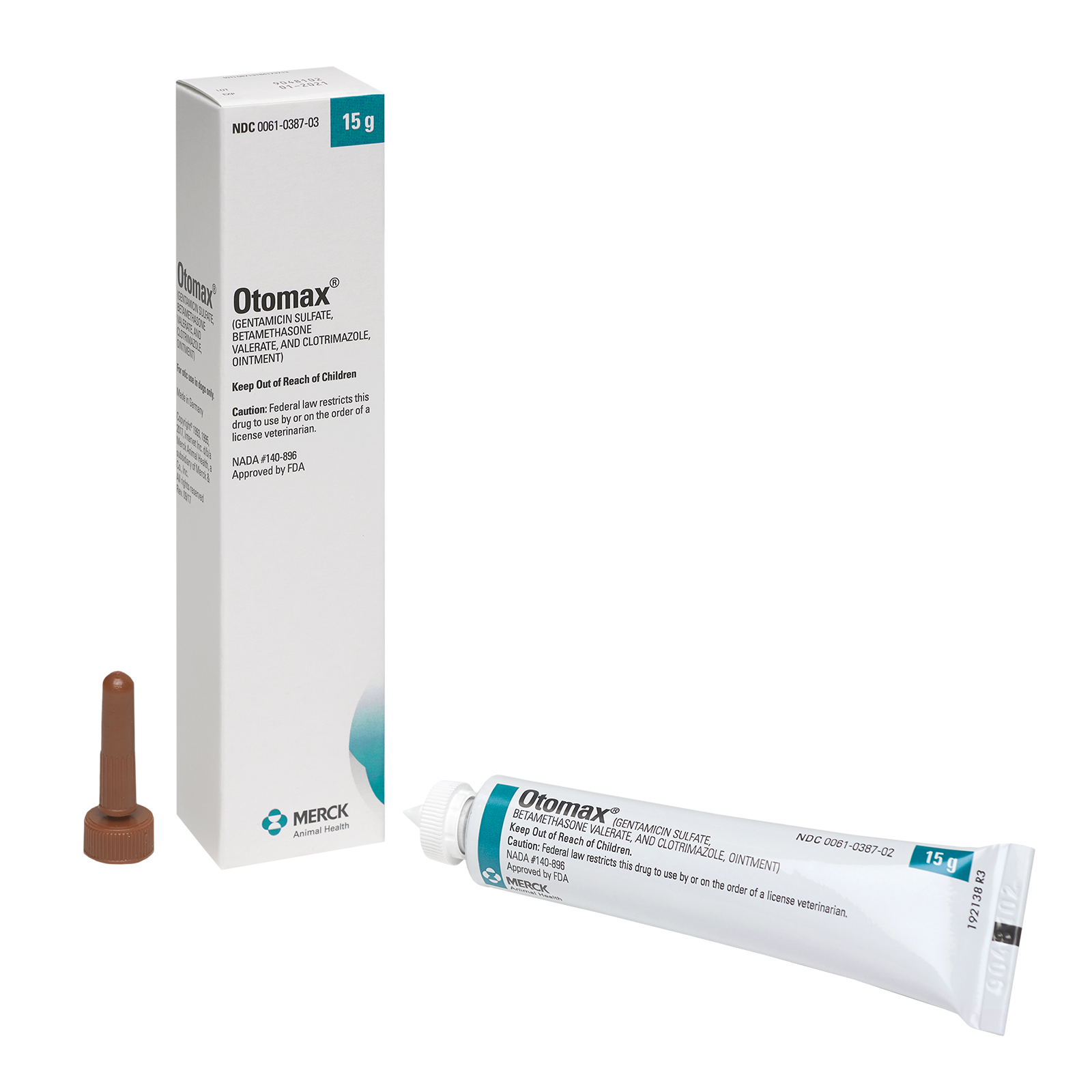
OTOMAX® Otic Ointment
(Gentamicin Sulfate, Betamethasone Valerate, and Clotrimazole, Ointment)
First-line power for acute and chronic cases
A powerful first-line, broad-spectrum topical medication administered twice a day for 7 days to treat otitis externa in dogs.

Indications
OTOMAX® Otic Ointment is indicated for the treatment of canine acute and chronic otitis externa associated with yeast (Malassezia pachydermatis) and/or bacteria susceptible to gentamicin.
Features & Benefits
OTOMAX is a trusted triple-action formula
ANTIBACTERIAL EFFECT
GENTAMICIN
Provides coverage against a wide variety of Gram-negative and Gram-positive bacteria, including Staphylococcus aureus, other Staphylococcus spp, and Pseudomonas aeruginosa
ANTI-INFLAMMATORY ACTION
BETAMETHASONE
A highly effective and safe corticosteroid that reduces inflammation
ANTIFUNGAL ACTIVITY
CLOTRIMAZOLE
Proven effective for the most common ear yeast isolate (Malassezia pachydermatis)
Diseases/Parasites
Description
Each gram of OTOMAX Otic Ointment contains gentamicin sulfate, equivalent to 3 mg gentamicin base; betamethasone valerate, equivalent to 1 mg betamethasone; and 10 mg clotrimazole, in a mineral oil-based system containing a plasticized hydrocarbon gel.
Administration and Dosage
For 7.5 g and 15 g tubes, as well as 15 g and 30 g bottles:
- Administer twice daily for 7 days
- 4 drops per ear for dogs < 30 lbs
- 8 drops per ear for dogs = or > 30 lbs
For 215 g bottles:
- Administer twice daily for 7 days
- 2 drops per ear for dogs < 30 lbs
- 4 drops per ear for dogs = or > 30 lbs
Vehicle
- Solution-like consistency with clear advantage
- Colorless solution is easy to differentiate from exudate
- Eliminates staining
- Hydrocarbon formulation consistency facilitates easy counting of drops for proper dosing
- Ensures prolonged contact at the site of infection
Supplied
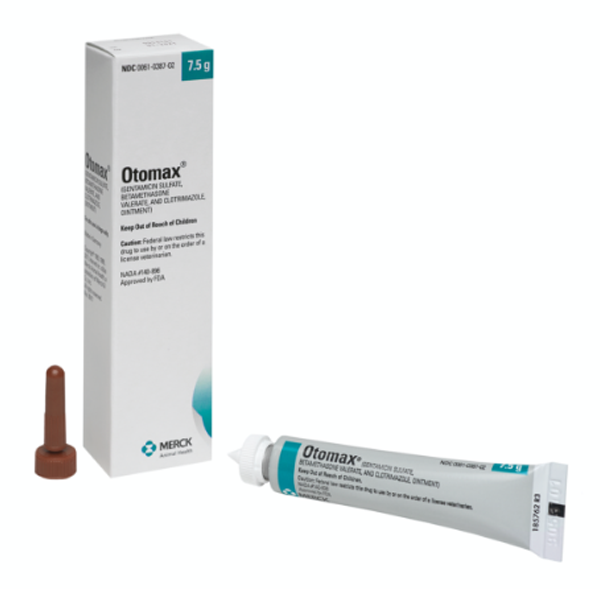
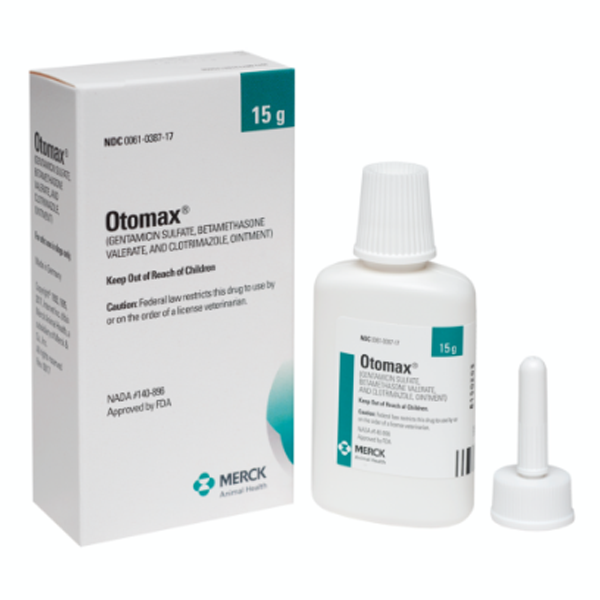
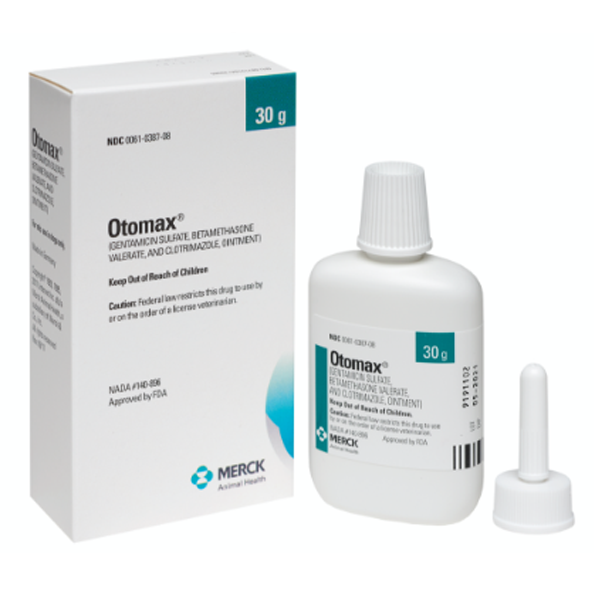
OTOMAX Otic Ointment is available in 7.5 g and 15 g tubes as well as 15 g, 30 g, and 215 g bottles.

Important Safety Information
Do not use in dogs with known perforation of eardrums. The use of OTOMAX® ointment has been associated with deafness or partial hearing loss in a small number of sensitive dogs (e.g., geriatric), though this is usually temporary. If hypersensitivity occurs, treatment should be discontinued. Concomitant use of drugs known to induce ototoxicity should be avoided. Administration of recommended doses beyond 7 days may result in delayed wound healing. Avoid ingestion. Keep out of the reach of children. For complete safety information, refer to the product label.
