TREMVAC®-FP-CAV
Avian Encephalomyelitis – Fowl Pox
& Chicken Anemia Virus Vaccine
Product Description
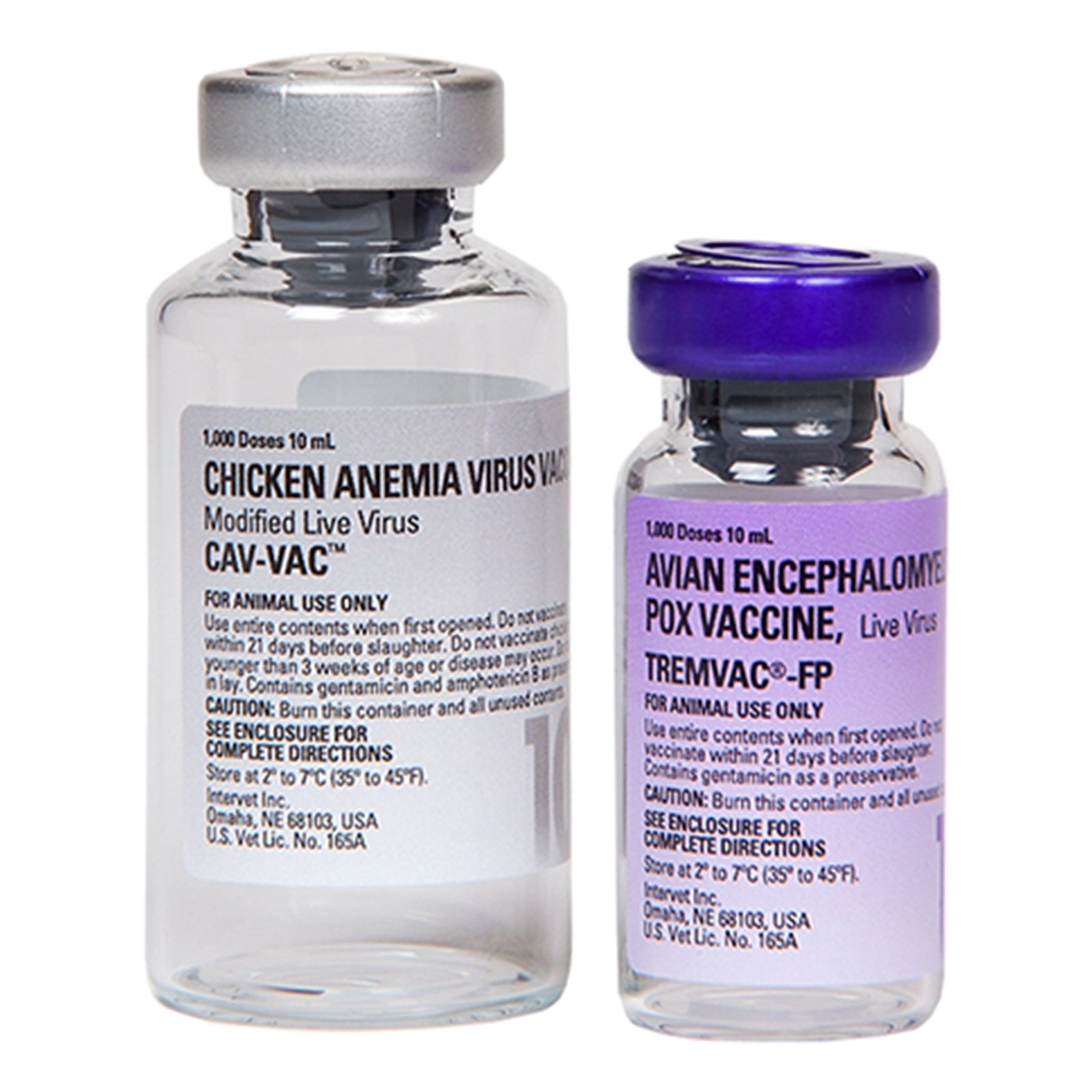
Tremvac-FP-CAV is a live virus vaccine containing avian encephalomyelitis virus prepared from the Calnek strain, a modified fowl pox virus, and chicken anemia virus prepared from a modified U.S. field isolate. The vaccine is packaged in two separate units. One is a vial containing freeze-dried avian encephalomyelitis virus and fowl pox virus (TREMVAC®-FP). The second is a vial containing chicken anemia virus suspension (CAV-VAC™) to be used as the wing web diluent.

Indications
This product has been shown to be effective for the vaccination of healthy chickens 10 to 12 weeks of age against avian encephalomyelitis, fowl pox disease and to provide passive immunity against chicken anemia virus to progeny.
Warning
WARNING: Use of this product in chickens younger than three weeks of age may cause clinical signs of chicken anemia. Do not vaccinate breeder chickens in lay.
Advantages*
- Excellent protection against infection with AE and fowl pox viruses
- The original Calnek strain of AE provides dependable immune response with consistent antibody titer
- Demonstrable efficacy with reliable development of “takes” at 7 days post-vaccination
- Induces high levels of protective antibodies for long-term protection of parent flocks and their progeny against CIA
- Provides uniform flock protection
- Combined product reduces vaccination costs
Diseases/Parasites
- Avian Encephalomyelitis (AE)
- Chicken Anemia Virus (CAV)
- Avipoxvirus – Fowlpox
Vaccination Programs
Many factors must be considered in determining the vaccination program for a particular farm or poultry operation. To be fully effective, the vaccine must be administered to healthy receptive birds held in proper environment under good management. In addition, the response may be modified by the age of the birds and their immune status. Seldom does 1 vaccination under field conditions produce complete protection for all individuals in a given flock. The amount of protection required will vary with the type of operation and the degree of exposure that a flock is likely to encounter.
Wing-Web Administration
FOR CHICKENS FROM 10 TO 12 WEEKS OF AGE
- Vaccine is applied to the web of the wing. Use the enclosed two-pronged applicator.
- A dose of 0.01 mL should be administered to each bird by dipping the applicator in the vaccine mixture, a lowing the applicator grooves to fill with liquid and stabbing the webbed portion of the wing from beneath. Avoid feathered areas of the web.
- Periodically during use, re-insert stopper and shake vaccine well.
- At about 7 to 10 days after vaccination, a few birds should be examined for takes. A good take reaction, indicating that a satisfactory vaccination job was done, shows swelling in the skin at the point of vaccination with scab formation. The scabs will fall off about 2 to 3 weeks following vaccination.
Supplied
10 x 1,000 Doses/vial Tremvac®-FP and
10 x 1,000 Doses (10 mL/vial) CAV-VAC® as diluent
Caution
- VACCINATE ONLY HEALTHY CHICKENS.
- All chickens should be vaccinated at the same time.
- Following vaccination, virus may be shed in the feces. Thus, care should be taken to avoid spread of the vaccine virus to young chickens or unexposed chickens in lay.
- Use entire contents when first opened.
- Do not vaccinate within 21 days before slaughter or 6 weeks prior to onset of or during lay.
- Store at 2° to 8°C (35° to 46°F). Do not freeze.
- Inactivate unused contents before disposal.
- Do not mix with other products, except as specified on this label.
- In case of human exposure, contact a physician.
- Contains gentamicin and amphotericin B as preservative.
- FOR ANIMAL USE ONLY.
Notice
This vaccine has undergone rigid potency, safety and purity tests, and meets Merck Animal Health, U.S. and local regulatory requirements. It is designed to stimulate effective immunity when used as directed, but the user must be advised that the response to the product depends upon many factors, including, but not limited to, conditions of storage and handling by the user, administration of the vaccine, health and responsiveness of the individual chickens and the degree of field exposure. Therefore, directions should be followed carefully. A safety data sheet (SDS) is available upon request. This and any other consumer information can be obtained by calling Merck Animal Health Customer Service at 1-800-211-3573.
Records
Keep a record of vaccine, quantity, serial number, expiration date, and place of purchase; the date and time of vaccination; the number, age, breed, and locations of chickens; names of operators performing the vaccination and any observed reactions.
Contact our sales or technical services representatives to help design a custom vaccination program.
Resources
For additional information, please see the product label.
For more information regarding efficacy and safety data, go to productdata.aphis.usda.gov.
Resources
*Data on file, Merck Animal Health

To view our complete product lineup, click to view our
POULTRY PRODUCT CATALOG vol. 3.1.