BOVILIS® VISTA® ONCE SQ
Bovine Rhinotracheitis-Virus Diarrhea-Parainfluenza 3-Respiratory Syncytial Virus-Mannheimia haemolytica-Pasteurella multicide Vaccine
Product Description
Get the most comprehensive coverage in an injectable respiratory vaccine. BOVILIS VISTA ONCE SQ gives you more coverage from the major causes of respiratory disease. It is proven effective against 6 viral causes and 2 bacterial causes including Pasteurella multocida and BVD Type 1b. It also has industry-leading duration of immunity.1-4

Key Benefits
- Shown to be effective for the vaccination of healthy cattle 3 months of age or older against:
- Respiratory disease and abortion due to infectious bovine rhinotracheitis (IBR)
- Respiratory disease and fetal infection, including persistently infected calves due to bovine virus diarrhea (BVD) Type 1, 1b and 2, bovine respiratory syncytial virus (BRSV). Parainfluenza 3 virus (PI3), Mannheimia haemolytica and Pasteurella multocida
- Shown to be effective for the vaccination of healthy cows and heifers prior to breeding:
- Against fetal infection, including persistently infected calves caused by BVD (Type 1, 1b and 2)
- Against persistently infected calves caused by BVD (Type 2)
- For reducing abortion due to IBR
- Industry-leading duration of immunity (DOI)1-4
- Respiratory DOI has been shown to be at least 1 year for IBR and BVD (Types 1 and 2) and at least 16 weeks for Mannheimia haemolytica and Pasteurella multocida
- Reproductive DOI has been shown to be at least 217 days for IBR, and at least 206 days for BVD (Types 1 & 2)
- Respiratory and reproductive efficacy and duration of immunity has been shown against disease caused by the BVD Type 1b strain
Dosage and Usage
- 2 mL administered subcutaneously to healthy cattle 3 months of age or older
- Annual revaccination is recommended
- Supplied in 10 and 50 dose vials
- Safe for use in pregnant heifers and cows or calves nursing pregnant cows provided the cows and heifers I the herd are vaccinated prior to breeding, within the previous 12 months
Resources
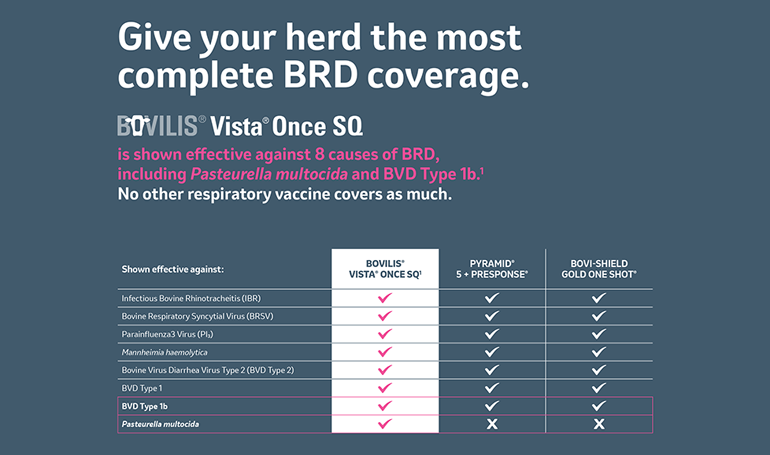
BOVILIS® VISTA® ONCE SQ Vaccine Comparison Chart
See how your vaccine compares in coverage.
No items to show.
Videos
BOVILIS® VISTA® : Addressing emerging diseases
A Game-changing Vaccine
Innovation is critical in vaccine development. Merck Animal Health has a history of finding a better way forward through game-changing advancements, and today offers one of the most complete vaccine portfolios. Learn about BOVILIS® VISTA® from members of the team who brought it to market.

Stay in front of profit-robbing diseases with the comprehensive cattle vaccine lineup from Merck Animal Health

See more solutions that can help improve herd health and boost performance for cow-calf and stocker operations.
Sign up to receive cattle health management insights, industry news and more sent straight to your inbox.
References
1. Purtle L, et al. One Year Duration of Immunity of the Modified Live Bovine Viral Diarrhea Virus Type 1 and Type 2 and Bovine Herpesvirus-1 Fractions of VISTA ONCE SQ VACCINE. Vaccine. 2016;34(13):1582-1588.
2. Report No. BRL-016R. Duration of Immunity in Calves for the Pasteurella multocida Fraction in Bovine Rhinotracheitis-Virus diarrhea Parainfluenza 3-Respiratory Syncytial Virus – Mannheimia haemolytica Pasteurella multocida Vaccine, Modified Live Virus, A Virulent Live Culture. August 23, 2013.
3. Platt R, Burdett W, Roth JA. Introduction of antigen-specific T-cell subset activation to bovine respiratory disease viruses by a modified-live virus vaccine. Am J Vet Res. 2006;67:1179-1184.
4. See product label for information on access to safety and efficacy study summaries.
