BOVILIS® NASALGEN® 3-PMH
Bovine Rhinotracheitis-Parainfluenza 3-Respiratory Syncytial Virus-Mannheimia Haemolytica-Pasteurella Multocida Vaccine
Product Description
The color of unmatched intranasal protection.
BOVILIS® NASALGEN® 3-PMH is the first and only intranasal BRD vaccine offering protection against IBR, BRSV, PI3, Pasteurella multocida and Mannheimia haemolytica. It’s safe to use in calves 1 week of age and older for a strong, healthy foundation. And a unique BluShadow® diluent means there’s no second-guessing which animals have been vaccinated.

Indications
This product has been shown to be effective for the vaccination of healthy cattle, 1 week of age or older against infectious Bovine Rhinotracheitis (IBR) virus, Bovine Respiratory Syncytial Virus (BRSV), Parainfluenza 3 virus (PI3), Mannheimia haemolytica and Pasteurella multocida. Duration of immunity against IBR is at least 195 days, against BRSV is at least 78 days, against PI3 is at least 95 days, against Mannheimia haemolytica is at least 122 days, and against Pasteurella multocida is at least 125 days.
Diseases
- Infectious bovine rhinotracheitis (IBR)
- Bovine respiratory syncytial virus (BRSV)
- Parainfluenze 3 (PI3)
- M. haemolytica
- P. multocida

Designed for no injection site reactions to help your operation meet Beef Quality Assurance standards.
Key Benefits
- Designed with IBR strains that are not temperature-sensitive.1 Ensures the vaccine will replicate and protect in any situation.
- Unique BluShadow® diluent for confident administration.
- Eliminates the need and stress of two separate vaccines.
- Safe in pregnant cows and in calves nursing pregnant cows.
Powerful, long-lasting duration of immunity2
- At least 195 days against IBR
- At least 95 days against PI3
- At least 78 days against BRSV
- At least 125 days against P. multocida
- At least 122 days against M. haemolytica
Usage
- Convenient, single 2 mL does in one nostril
- Mimics natural exposure to the most common causes of pneumonia for an effective immune response
A Game-changing Vaccine
Innovation is critical in vaccine development. For decades it was believed that vaccinating young animals was not effective because of maternal antibody interference. The industry needed to find a way to protect young calves from BRD.
Learn from members of the team who brought to market BOVILIS NASALGEN 3-PMH – the first and only intranasal BRD vaccine offering protection against viral and bacterial pathogens.

Don’t leave a gap in your protection
In addition to BRD, bovine viral diarrhea (BVD) is widespread, serious and costly. And most herds are at risk.
By combining all the benefits of BOVILIS NASALGEN 3-PMH with the added BVD coverage of BOVILIS® VISTA® BVD CFP, you can get calves off to a strong start.
No other combination offers more when it comes to coverage and duration.
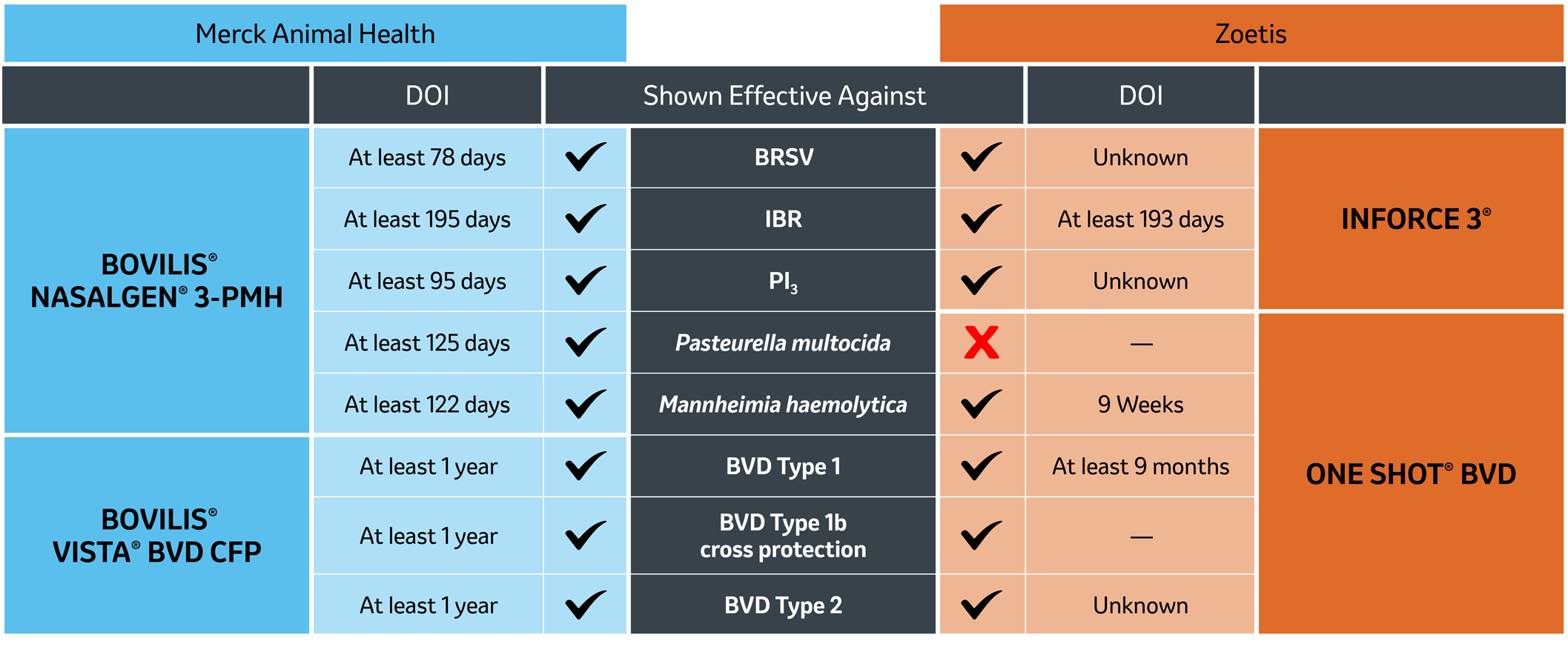



provides the most complete coverage – proven effective against both BVD Type 1b and
Pasteurella multocida – that the Zoetis® option lacks.
- BVD Type 1b accounts for more than two-thirds of BVD-positive cattle – duration and cross protection are key as maternal antibody protection wears off.3
- Pasteurella multocida is the second most isolated bacteria recovered from lung samples at Midwestern diagnostics labs – and young calves are especially susceptible.4
The first and only intranasal vaccine with viral and bacterial pneumonia protection.
That means protection against IBR, BRSV, PI3, Pasteurella multocida and Mannheimia haemolytica. BOVILIS NASALGEN 3-PMH is also safe to use in calves 1 week of age or older for a strong, healthy foundation. And a unique BluShadow® diluent means there’s no second-guessing which animals have been vaccinated.
Dosage
Shake well and administer a 2 mL dose by the intranasal route. For advice on revaccination frequency, contact your veterinarian.
Supplied
20 mL x 10 doses (2 mL per dose)
25 mL x 1 dose/2 mL x 25 doses (2mL per dose)
100 mL x 50 doses (2 mL per dose)
Caution
Store at 2 to 8°C (35 to 46°F). Fetal health risks associated with the vaccination of pregnant animals with this vaccine cannot be unequivocally determined during clinical trials conducted for licensure. Appropriate strategies to address the risks associated with vaccine use in pregnant animals should be discussed with a veterinarian. Use entire contents when first opened. Do not use chemical disinfectants to sterilize syringes or needles. Do not mix with other products, except as specified on the label. Inactivate unused contents before disposal. Do not vaccinate within 21 days of slaughter. Anaphylactoid reactions may occur following use. Antidote: Epinephrine. Contains Penicillin and Streptomycin as a preservative. In case of human exposure, contact a physician.
FOR ANIMAL USE ONLY.
Research
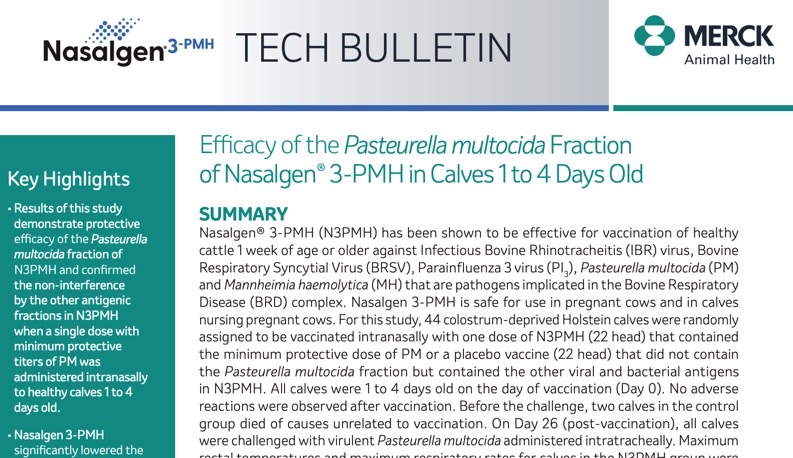
Efficacy of the Pasteurella multocida Fraction of BOVILIS® Nasalgen® 3-PMH in Calves 1 to 4 Days Old
Results of this study demonstrate protective efficacy of the Pasteurella multocida fraction of BOVILIS® NASALGEN® 3-PMH.
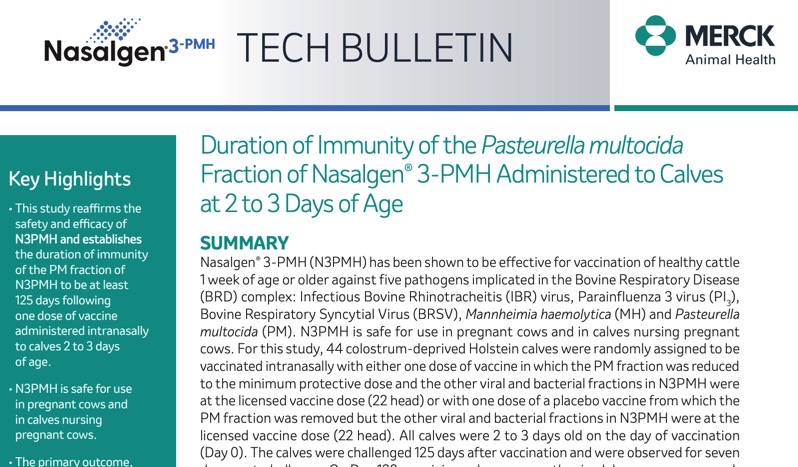
Duration of Immunity of the Pasteurella multocida Fraction of BOVILIS® Nasalgen® 3-PMH Administered to Calves at 2 to 3 Days of Age
Results of this study demonstrate the duration of immunity of the Pasteurella multocida fraction of BOVILIS® NASALGEN® 3-PMH.
Efficacy of the Mannheimia haemolytica Fraction of BOVILIS® Nasalgen® 3-PMH in Calves 2 to 4 Days Old
Results of this study demonstrate protective efficacy of the Mannheimia haemolytica fraction of BOVILIS® NASALGEN® 3-PMH.
Duration of Immunity of the Mannheimia haemolytica Fraction of BOVILIS® Nasalgen® 3-PMH Administered to Calves 4 to 6 Days of Age
Results of this study demonstrate the duration of immunity of the Mannheimia haemolytica fraction of BOVILIS® NASALGEN® 3-PMH.

Efficacy of the Bovine Respiratory Syncytial Virus Fraction of BOVILIS® Nasalgen® 3-PMH in Calves 4 to 7 Days Old
Significant differences in lung lesion scores and viral shedding between BOVILIS® Nasalgen 3-PMH® vaccinated and control groups confirmed the immunogenic efficacy of the BRSV fraction of N3PMH.

Duration of Immunity of the Bovine Respiratory Syncytial Virus Fraction of BOVILIS® Nasalgen® 3-PMH Administered to Calves 5 to 7 Days of Age
This study reaffirms the safety and efficacy of N3PMH and establishes the duration of immunity of the BRSV fraction of N3PMH to be at least 78 days following one dose of vaccine administered intranasally to calves 5 to 7 days of age.
Efficacy of the Infectious Bovine Rhinotracheitis Fraction of BOVILIS® Nasalgen® 3-PMH in Calves 4 to 7 Days Old
Results of this study demonstrate protective efficacy for the IBR viral fraction of Nasalgen 3-PMH and no interference by the other four antigens in N3PMH after one intranasal administration to calves 4 to 7 days old.
Duration of Immunity of the Infectious Bovine Rhinotracheitis Virus Fraction of BOVILIS® Nasalgen® 3-PMH Administered to Calves 3 to 5 Days of Age
This study reaffirms the safety and efficacy of N3PMH and establishes the duration of immunity of the IBR fraction of N3PMH to be at least 195 days following one dose of vaccine administered intranasally to calves 3 to 5 days of age.

Efficacy of the Bovine Parainfluenza 3 Virus Fraction of BOVILIS® Nasalgen® 3-PMH in Calves 6 or 7 Days Old
Results of this study demonstrate protective efficacy of the PI3 fraction of BOVILIS® Nasalgen 3-PMH and confirmed the non-interference by the other antigenic fractions in N3PMH when administered intranasally to healthy calves 6 or 7 days old.

Duration of Immunity of the Parainfluenza 3 Fraction of BOVILIS® Nasalgen® 3-PMH Administered to Calves 3 to 5 Days of Age
This study reaffirms the safety and efficacy of N3PMH and establishes the duration of immunity of the PI3 fraction of N3PMH to be at least 95 days following one dose of vaccine administered intranasally to calves 3 to 5 days of age.
No items to show.
Contact
U.S. only: Merck Animal Health contact us or call 1-800-211-3573.
For more information regarding efficacy and safety data, go to productdata.aphis.usda.gov
For additional information, please see the product label.
References
1. Grissett GP, et al. Effect of Ambient Temperature on Viral Replication and Serum Antibody Titers Following Administration of a Commercial Intranasal Modified-Live Infectious Bovine Rhinotracheitis-Parainfluenza-3 Virus Vaccine to Beef Cattle Housed in High- and Moderate-Ambient Temperature Environments. Am J Vet Res. 2014;75(12):1076-1082.
2. See Product Label.
3. Meyer B, Hill K, Burdett B, Engelken TJ, Roth J, Renter D. et al. Haptoglobin Study of Vista in young beef calves. Bov Pract. 2014.
4. Andersen PH. Bovine Endotoxin. Some aspects of relevance to production diseases. A Review. Acta Vet Scand Suppl. 2003;98:141-155.

For more cattle-friendly options, see the rest of the Merck Animal Health vaccine lineup.

Sign up to receive cattle health management insights,
industry news and more sent straight to your inbox.
