


Treatment & dosing
Treating bovine respiratory disease and controlling respiratory disease in cattle at high risk shouldn’t be difficult.
Administering long-lasting ZUPREVO® (tildipirosin) is easy.

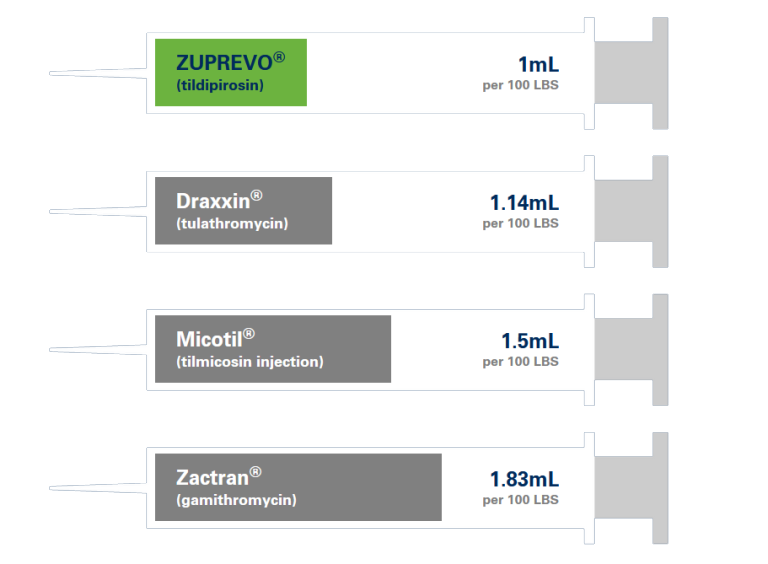
ONE LOW-VOLUME DOSE
Helps you get more doses per bottle.

1 ML/
100 LBS
Easy to calculate. Easy to remember.

HIGHLY SYRINGEABLE
Easy to use in any weather conditions.

FLEXIBLE
TIMING
21-day meat withdrawal.
See the difference one dose can make.
Get the most from every mL.
Compared to other popular BRD products, ZUPREVO helps you get more doses from every bottle.
All trademarks are property of their respective owners.
Get the BRD treatment that packs efficiency and convenience in every dose.
ZUPREVO™ 18%
(Tildipirosin)
Product Description
Injectable Solution for Cattle
ZUPREVO is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida and Histophilus somni. ZUPREVO is also indicated for the reduction of morbidity associated with BRD in feedlot calves, caused by Mannheimia haemolytica, Pasteurella multocida and Histophilus somni, during the first 14 days in the feedlot, when administered at the time of arrival.

Indications
Zuprevo™ 18% is indicated for the treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni in beef and non-lactating dairy cattle, and for the control of respiratory disease in beef and non-lactating dairy cattle at high risk of developing BRD associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni.
For subcutaneous injection in beef and non-lactating dairy cattle only.
Not for use in female dairy cattle 20 months of age or older or in calves to be processed for veal.
Caution
Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Indicated Pathogens
- Mannheimia haemolytica
- Pasteurella multocida
- Histophilus somni
Dosage
Inject subcutaneously as a single dose in the neck at a dosage of 4 mg/kg (1 mL/100 lb) body weight (BW). Do not inject more than 10 mL per injection site. Do not puncture the stopper of the respective vial size more than the tested number of punctures, shown in Table 1.
Clinical field studies indicate that administration of Zuprevo™ 18% (tildipirosin) Injectable Solution is effective for the control of respiratory disease in beef and non-lactating dairy cattle at “high risk” of developing BRD. Calves at high risk of developing BRD typically experience one or more of the following risk factors:
- Commingling from multiple sale barns/sources
- Extended transport times and shrink
- Exposure to wet or cold weather conditions or wide temperature swings
- Stressful arrival processing procedures (such as castration, dehorning, or branding)
- Recent weaning and poor vaccination history
Table 1 Number of punctures tested in the in-use study for the respective vial sizes
| Vial size [mL] | Number of punctures tested in the in-use study |
|---|---|
| 50 | 8 |
| 100 | 8 |
| 250 | 16 |
Precautions
The effects of Zuprevo™ 18% on bovine reproductive performance, pregnancy and lactation have not been determined. Swelling and inflammation, which may be severe, may be seen at the injection site after administration. Subcutaneous injection may result in local tissue reactions which persist beyond the slaughter withdrawal period. This may result in trim loss of edible tissue at slaughter.
Effectiveness
In a multi-location field study, calves with naturally occurring BRD were treated with tildipirosin. The treatment success rate of the tildipirosin-treated group was compared to the treatment success rate in the saline-treated control group. A treatment success was defined as a calf not designated as a treatment failure from Day 1 to 13 and with normal attitude, normal respiration, and a rectal temperature of < 104°F on Day 14. The treatment success rate was significantly higher (p = 0.003) for the tildipirosin-treated group (229/300, 76%) compared to the saline-treated control group (96/200, 32%). There were no BRD-related deaths in the tildipirosin-treated group compared to a 7% (21/300) BRD-related mortality rate in the saline-treated group.
In another multi-location field study, calves at high risk for developing BRD were administered tildipirosin. The treatment success rate of the tildipirosin-treated group was compared to the treatment success rate in the saline-treated control group. A treatment success was defined as a calf not designated as a treatment failure based on clinical respiratory and attitude scoring and, if necessary, rectal temperature measurement of < 104°F through the end of the study (Day 14). The treatment success rate was significantly higher (p = 0.0001) for the tildipirosin-treated group (305/386, 79%) compared to the saline-treated group (197/387, 51%). There were three BRD-related deaths during the study (one tildipirosin-treated calf and two saline treated calves).
Safety
The macrolide with a high margin of safety.
As a new chemical entity, ZUPREVO antibiotic establishes a level of animal and consumer safety.
Human Safety
Caution should be taken to avoid accidental self injection. In the case of human injection, seek medical advice immediately and show the package insert or label to the physician. Do not use in automatically powered syringes (such as air or electrically powered syringes) that have no additional protection system.
Avoid direct contact with skin and eyes.
IF EYE EXPOSURE
- Rinse eyes with clean water.
IF SKIN EXPOSURE
- Wash the skin immediately with soap and water
- Wash hands after use.
- Tildipirosin may cause sensitization by skin contact.
Animal Safety
A target animal safety study was conducted using Zuprevo™ 18% administered in 5-month-old cattle as three subcutaneous doses of 4, 12, or 20 mg/kg BW given 7 days apart (1X, 3X, and 5X the labeled dose). Animals remained clinically healthy during the study at the labeled dose. Injection site swelling and inflammation, initially severe in some animals, was observed that persisted to the last day of observation (21 days after injection). No other drug-related lesions were observed macroscopically or microscopically at the labeled dose.
A separate injection site tolerance study was conducted using Zuprevo™ 18% in 5- to 9-month-old cattle administered as a single subcutaneous injection of 10 mL. Injection site swelling and inflammation, initially severe in some animals, was observed that persisted to the last day of observation (35 days after injection). No other drug-related clinical signs were observed.
Supplied
50 mL, amber glass, sterile, multi-dose vials.
100 mL, amber glass, sterile, multi-dose vials.
250 mL, amber glass, sterile, multi-dose vials.
Storage And Disposal
Do not store above 30°C (86°F). Do not freeze. The maximum storage time after first puncture is 28 days at or below 25°C (77°F).
Residue Warning
Cattle intended for human consumption must not be slaughtered within 21 days of the last treatment. Do not use in female dairy cattle 20 months of age or older. Use of this drug product in these cattle may cause milk residues. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal.
Important Safety Information
Zuprevo: FOR USE IN ANIMALS ONLY. NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. TO AVOID ACCIDENTAL INJECTION, DO NOT USE IN AUTOMATICALLY POWERED SYRINGES WHICH HAVE NO ADDITIONAL PROTECTION SYSTEM. IN CASE OF HUMAN INJECTION, SEEK MEDICAL ADVICE IMMEDIATELY AND SHOW THE PACKAGE INSERT OR LABEL TO THE PHYSICIAN. Cattle intended for human consumption must not be slaughtered within 21 days of the last treatment. Do not use in female dairy cattle 20 months of age or older. Use of this drug product in these cattle may cause milk residues. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal. The effects of Zuprevo®18% on bovine reproductive performance, pregnancy and lactation have not been determined. Swelling and inflammation, which may be severe, may be seen at the injection site after administration. Subcutaneous injection may result in local tissue reactions which persist beyond slaughter withdrawal period. This may result in trim loss of edible tissue at slaughter.
DO NOT USE Zuprevo®18% IN SWINE. Fatal adverse events have been reported following the use of tildipirosin in swine. NOT FOR USE IN CHICKENS OR TURKEYS.
Contact
U.S. only: Merck Animal Health livestocktechsrvc@merck-animal-health.com or call 1-800-211-3574
For additional information, please see the product label.

Get the latest updates! Sign up to receive cattle health management insights, industry news and more sent straight to your inbox.
